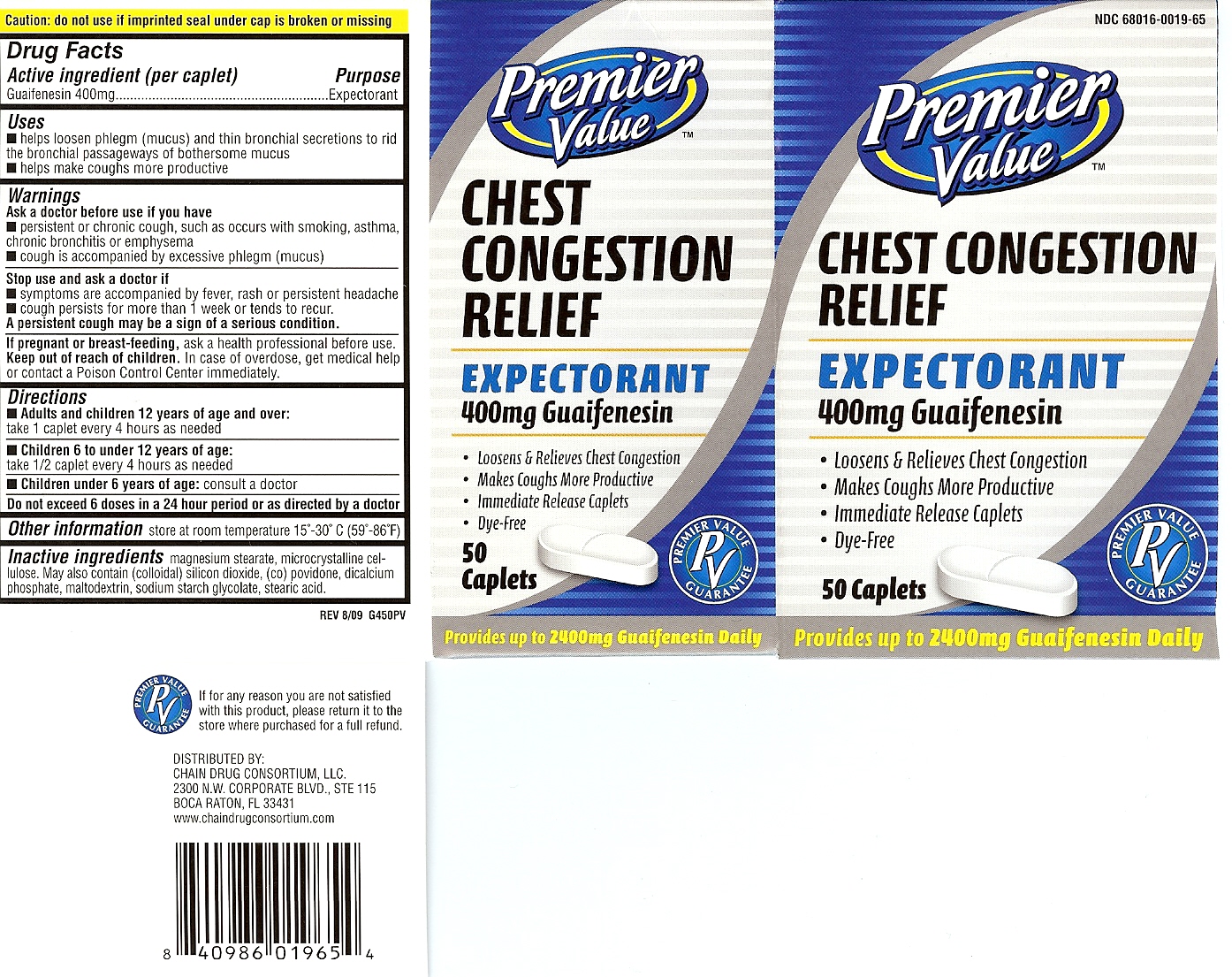
helps loosen phlegm (mucus) and thin
bronchial secretions to rid the bronchial passageways of bothersome mucus
• helps make coughs more productive
Ask doctor before use if you have
persistent or chronic cough, such as occurs with smoking, asthma, bronchitis or emphysema
cough is accompanied by excessive phlegm (mucous)
Keep out of reach of children
In case of overdose,get medical help or contact a poison control
cenetr immediately.
Stop use and ask doctor if
Symptoms are accompanied by fever, rash or persistent headache
cough persists for more than 1 week or tends to recur
A persistent cough may be a sign of a serious condition
Directions
• Adults and children 12 years of age and over:
take 1 tablet every 4 hours as needed
• Children 6 to 10 under 12 years of age: take 1/2 tablet every 4 hours as needed
• Children under 6 years of age: consult a doctor
Do not exceed 6 doses in a 24 hour period or as directed by a doctor