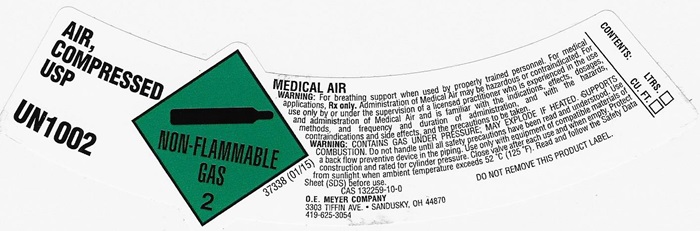
AIR, COMPRESSED LABEL
AIR, COMPRESSED USP UN1002 NON-FLAMMABLE GAS 2 37338 (01/15)
MEDICAL AIR
WARNING: For breathing support when used by properly trained personnel. For medical applications. Rx only. Administration of Medical Air may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of Medical Air and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications and side effects and the precautions to be taken.
WARNING: CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED, SUPPORTS COMBUSION. Do not handle until all safety precautions have been read and understood. Use a backflow preventive device I the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.
CAS 132259-10-0 DO NOT REMOVE THIS PRODUCT LABEL.
CONTENTS: LTRS. _______
CU. FT. _______
O.E. MEYER COMPANY
3303 TIFFIN AVE. • SANDUSKY, OH 44870
419-6253054