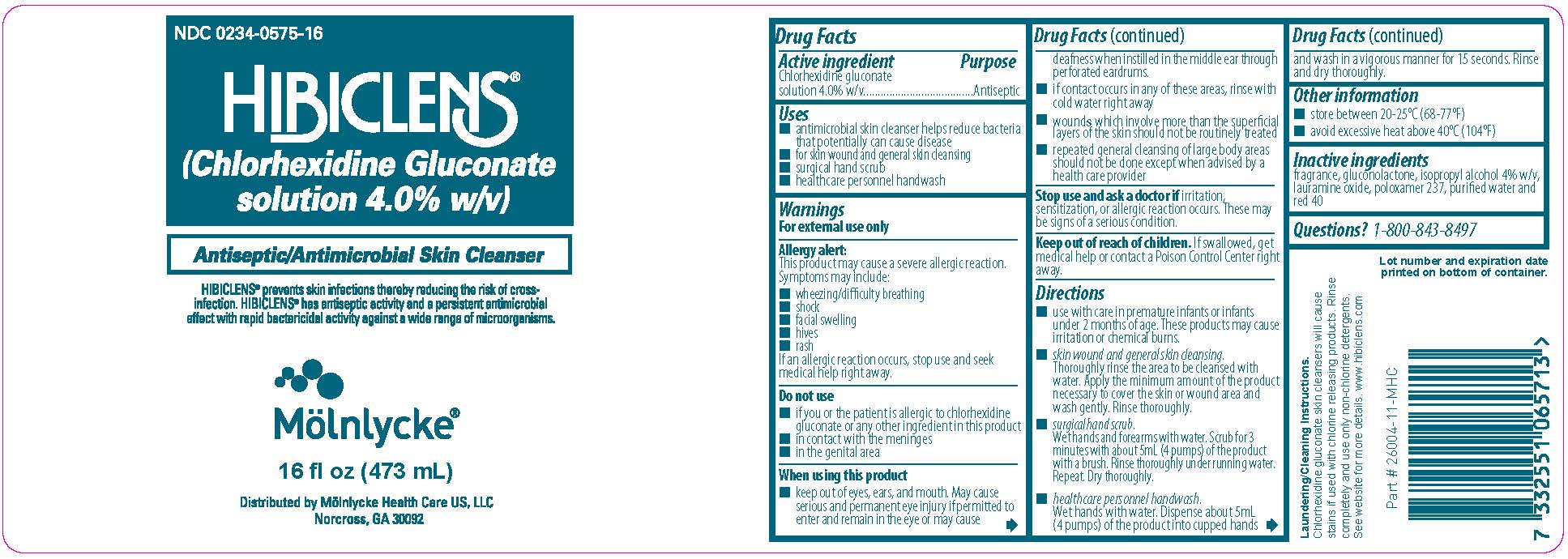
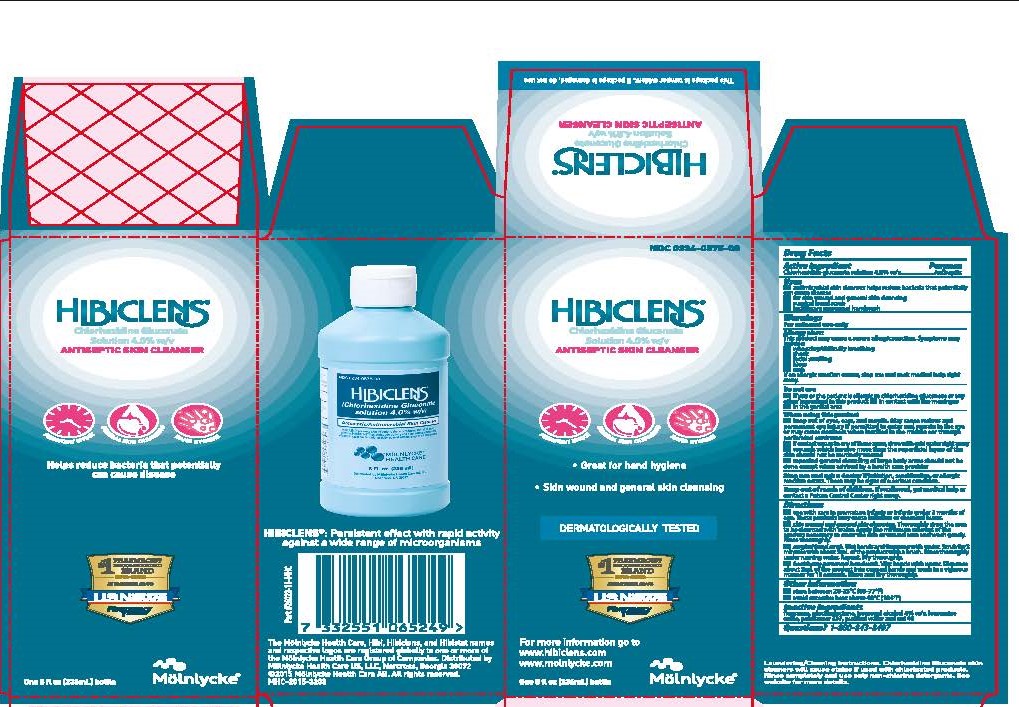
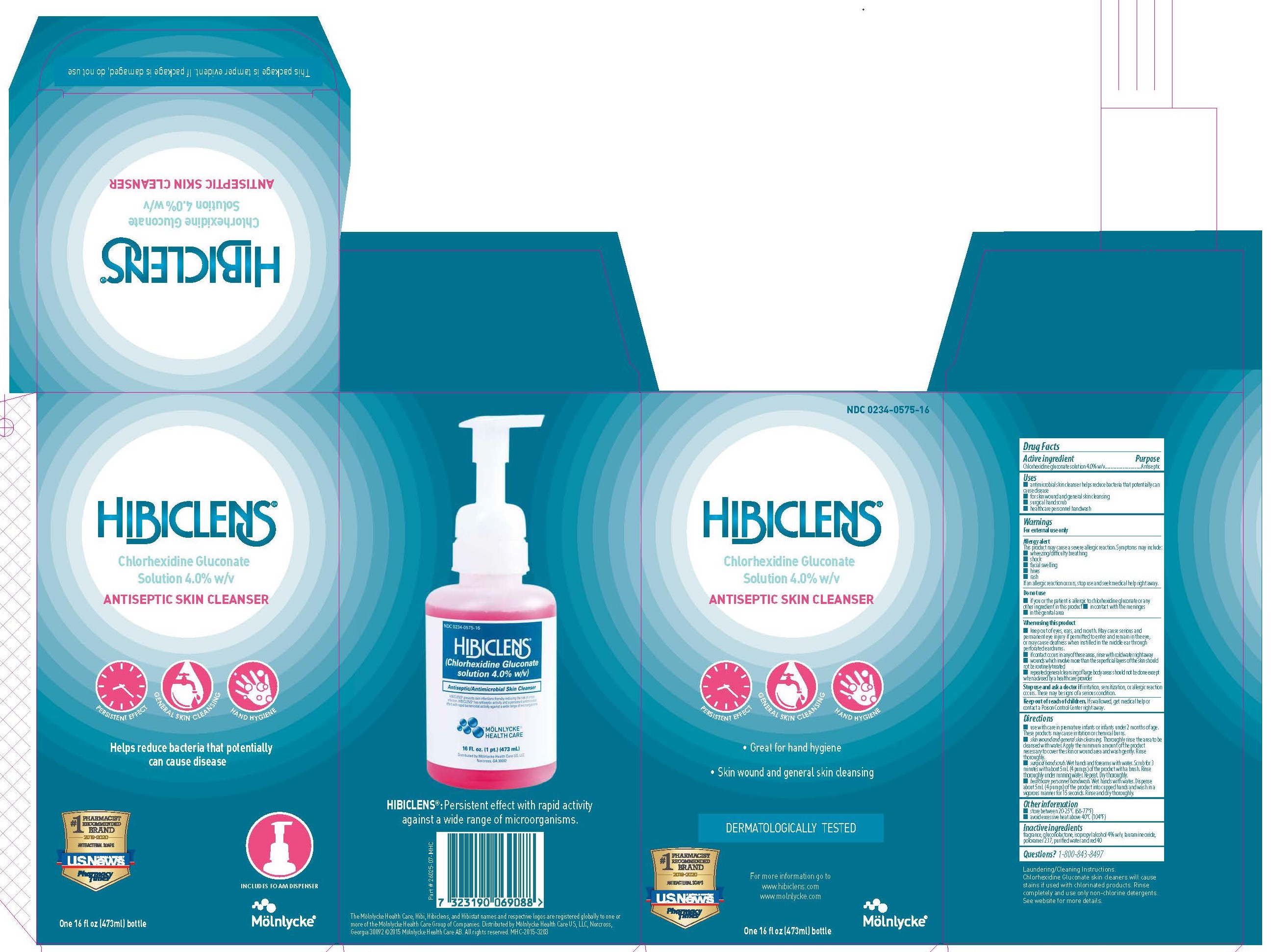
Uses
- antimicrobial skin cleanser helps to reduce bacteria that potentially can cause disease
- for skin wound and general skin cleansing
- surgical hand scrub
- healthcare peronnel handwash
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use
- if you or the patient is allergic to chlorhexidine gluconate or any other ingredient in this product
- in contact with the meninges
- in the genital area
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums
- if contact occurs in any of these areas, rinse with cold water right away
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general cleansing of large body areas should not be done except when advised by a health care provider
Stop use and ask a doctor if
irritation, sensitization, or allergic reaction occurs. These may be signs of a serious condition.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- skin wound and general skin cleansing. Thoroughly rinse the area to be cleansed with water. Apply the minimum amount of the product necessary to cover the skin or wound area and wash gently. Rinse thoroughly.
- surgical hand scrub. Wet hands and forearms with water. Scrub for 3 minutes with about 5mL of the product with a brush. Rinse thoroughly under running water. Repeat. Dry thoroughly.
- healthcare personnel handwash. Wet hand with water. Dispense about 5mL of the product into cupped hands and wash in a vigorous manner for 15 seconds. Rinse and dry thoroughly.