NARAMIN - diphenhydramine hydrochloride solution
National Pharma Industries Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
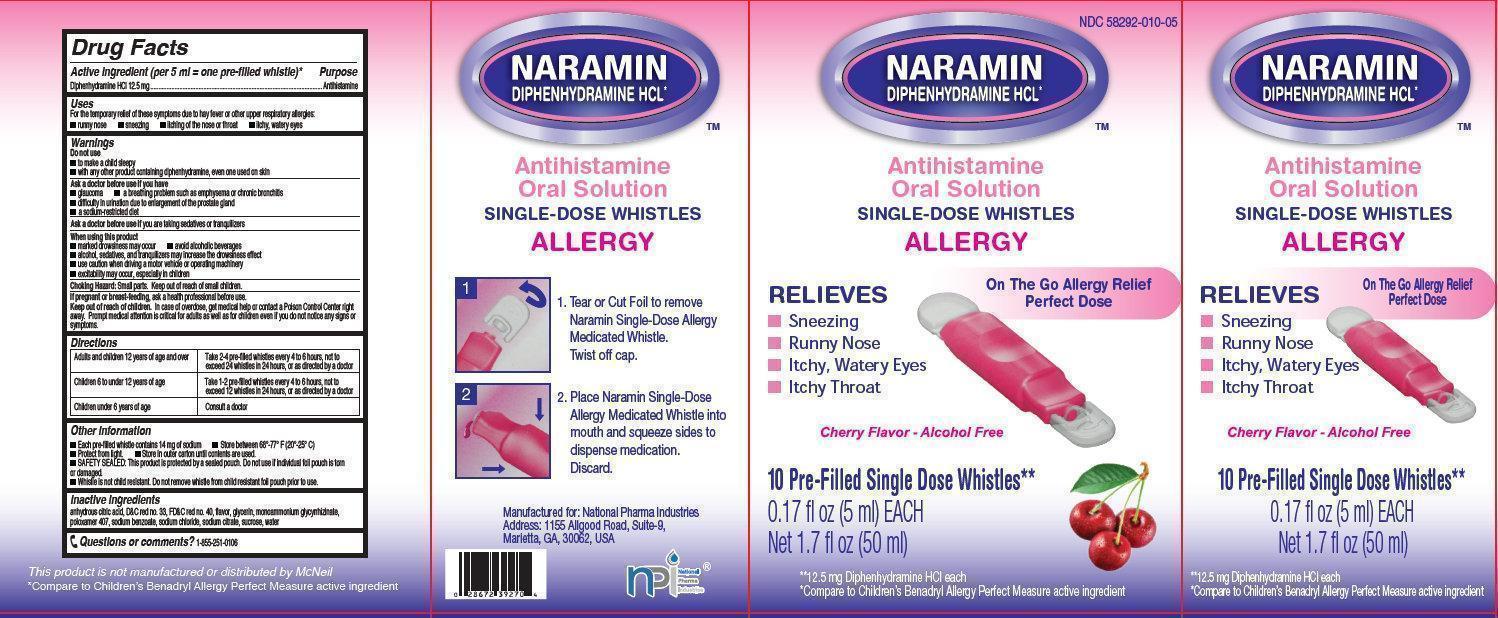
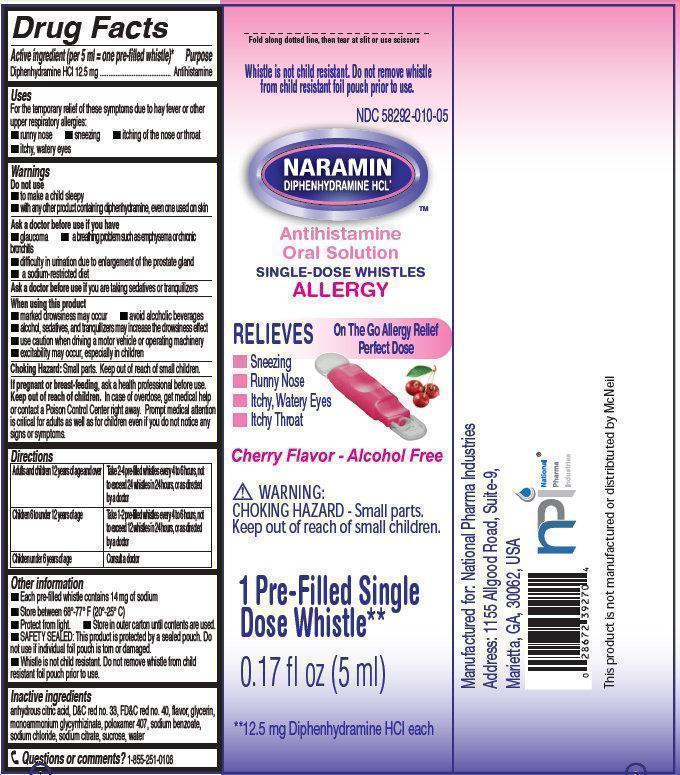
NARAMIN Antihistamine Oral Solution
Active ingredient (per 5 ml = one pre-filled whistle)*
Diphenhydramine HCl 12.5 mg
Uses
For the temporary relief of these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a sodium-restricted diet
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you
are taking sedatives or tranquilizers
When using this product
marked drowsiness may occur
avoid alcoholic beverages
alcohol, sedatives, and tranquilizers may increase the drowsiness effect
use caution when driving a motor vehicle or operating machinery
excitability may occur, especially in children
Choking Hazard: Small parts. Keep out of reach of children.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
|
|
|
Adults and children 12 years of age and over
|
Take 2-4 pre-filled whistles every 4 to 6 hours, not to exceed 24 whistles in 24 hours, or as directed by a doctor
|
|
Children 6 to under 12 years of age
|
Take 1-2 pre-filled whistles every 4 to 6 hours, not to exceed 12 whistles in 24 hours, or as directed by a doctor
|
|
Children under 6 years of age
|
Consult a doctor
|
Other information
- Each pre-fille whistle contains 14 mg of sodium
- Protect from light.
- SAFETY SEALED: This product is protected by a sealed pouch. Do not use if individual foil pouch is torn or damaged.
- Whistle is not child resistant. Do not remove whistle from child resistant foil pouch prior to use.
- Store between 68 degrees-77 degrees F (20 degrees - 25 degrees C)
- Store in outer carton until contents are used.
Inactive ingredients
anhydrous citric acid, DandC red no. 33, FDandC red no.40, flavor, glycerin, monoammonium glycyrrhizinate, poloxamer 407, sodium benzoate, sodium chloride, sodium citrate, sucrose, water
Questions or comments?
1-855-251-0106
NARAMIN Antihistamine Oral Solution 10-5ml whistles (58292-010-05)