Warnings
For external use only.
Do not use if pouch is damaged or opened. Do not use
- •
- on large areas of the body or on cut, irritated skin
- •
- on open wounds or sensitive skin
- •
- for more than one week without consulting a doctor
- •
- do not use if you are allergic to lidocaine or any of the inactive ingredients listed below.
When using this product
- •
- use only as directed. Read and follow all directions and warnings on this package.
- •
- do not allow contact with the eyes
- •
- do not bandage or apply local heat (such as heating pads) to the area of use
- •
- do not reuse patch.
Directions
Adults and children over 12 years of age: Apply patch to affected area every 8 to 12 hours.
Children 12 years or younger: Consult a physician before use.
Instructions for Use
- •
- clean and dry affected area
- •
- cut open pouch and remove the patch
- •
- remove clear protective liner from the patch
- •
- apply to affected area no more than three (3) times per day
- •
- leave in place for up to 8 but no more than 12 hours
- •
- wash hands thoroughly after applying or removing patch
- •
- some individuals may not experience pain relief until several minutes or hours after applying a patch
- •
- do not use other local anesthetic products in addition to Lidocare.
Inactive Ingredients
Crospovidone, menthoxypropanediol, non-woven backing fabric, polyester film, non-latex rubber based adhesive, vanillyl butyl ether.
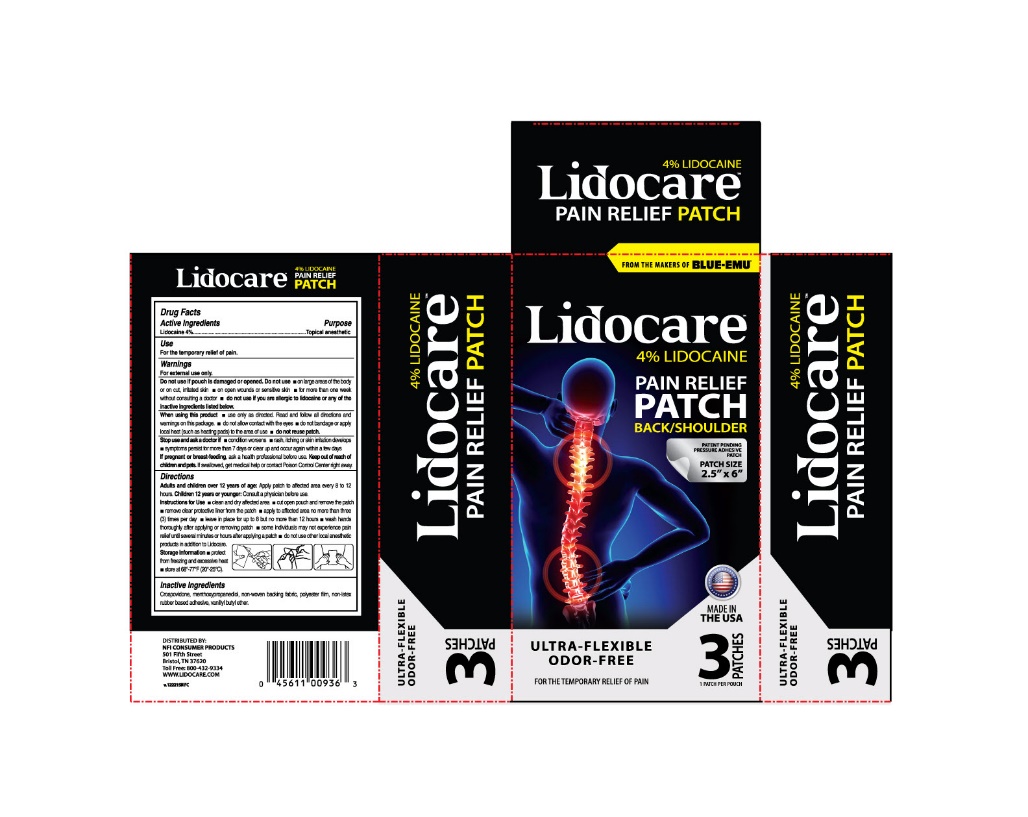
PRINCIPAL DISPLAY PANEL – 3 Patch Carton Label
FROM THE MAKERS OF BLUE-EMU®
Lidocare™
4% LIDOCAINE
PAIN RELIEF
PATCH
BACK/SHOULDER
PATENT PENDING
PRESSURE ADHESIVE
PATCH
PATCH SIZE
2.5” x 6”
MADE IN
THE USA
ULTRA-FLEXIBLE
ODOR-FREE
FOR THE TEMPORARY RELIEF OF PAIN
3 PATCHES
1 PATCH PER POUCH
DISTRIBUTED BY:
NFI CONSUMER PRODUCTS
501 Fifth Street
Bristol, TN 37620
Toll Free: 800-432-9334
WWW.LIDOCARE.COM