PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1173-1 in bottle of 100 Tablets
Amitriptyline Hydrochloride Tablets USP, 10 mg
Rx only
100 Tablets

NDC 70771-1174-1 in bottle of 100 Tablets
Amitriptyline Hydrochloride Tablets USP, 25 mg
Rx only
100 Tablets

NDC 70771-1175-1 in bottle of 100 Tablets
Amitriptyline Hydrochloride Tablets USP, 50 mg
Rx only
100 Tablets

NDC 70771-1176-1 in bottle of 100 Tablets
Amitriptyline Hydrochloride Tablets USP, 75 mg
Rx only
100 Tablets

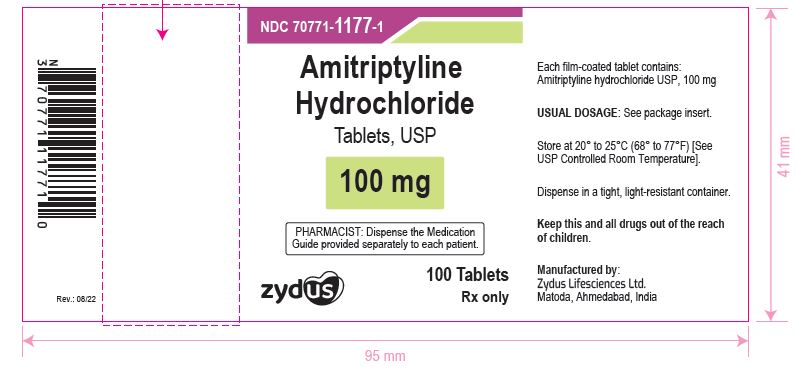
NDC 70771-1177-1 in bottle of 100 Tablets
Amitriptyline Hydrochloride Tablets USP, 100 mg
Rx only
100 Tablets
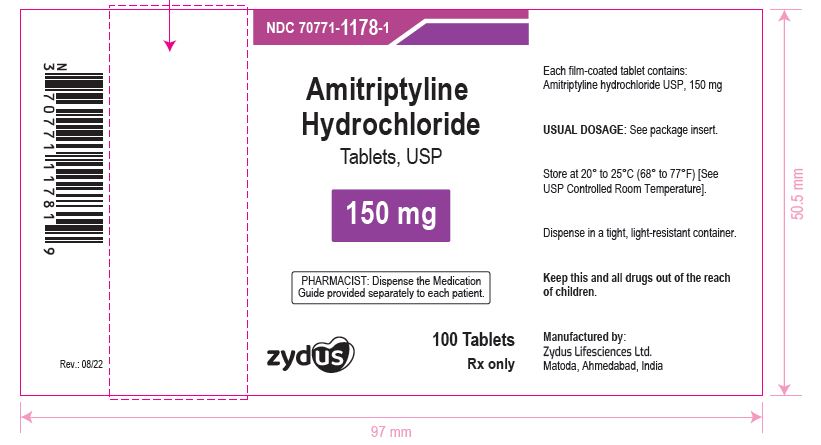
NDC 70771-1178-1 in bottle of 100 Tablets
Amitriptyline Hydrochloride Tablets USP, 150 mg
Rx only
100 Tablets