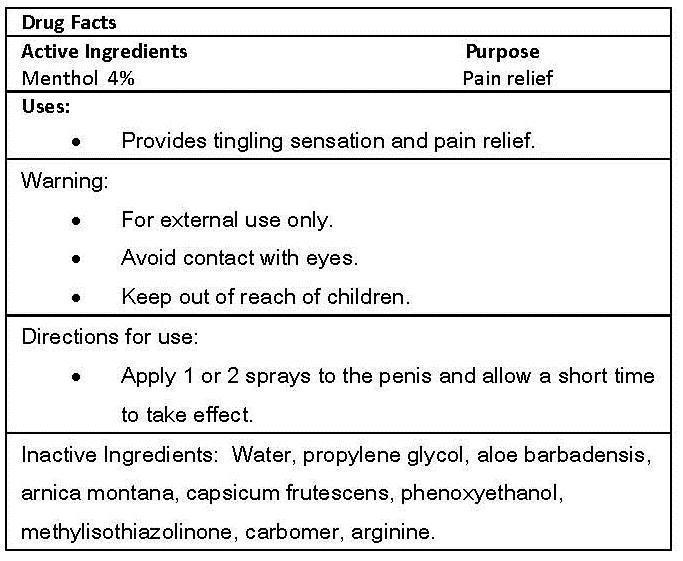
EROS IRON MAN- menthol spray
MEGASOL COSMETIC GMBH
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| EROS IRON MAN
menthol spray |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - MEGASOL COSMETIC GMBH (344152855) |
| Registrant - MEGASOL COSMETIC GMBH (344152855) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MEGASOL COSMETIC GMBH | 344152855 | manufacture(53389-039) | |
Revised: 1/2018
Document Id: 885194d9-985c-4aad-b36a-8706fa1b0132
Set id: 40fcb1b4-65a3-45d9-95b7-ca58c4bd741d
Version: 3
Effective Time: 20180116
MEGASOL COSMETIC GMBH