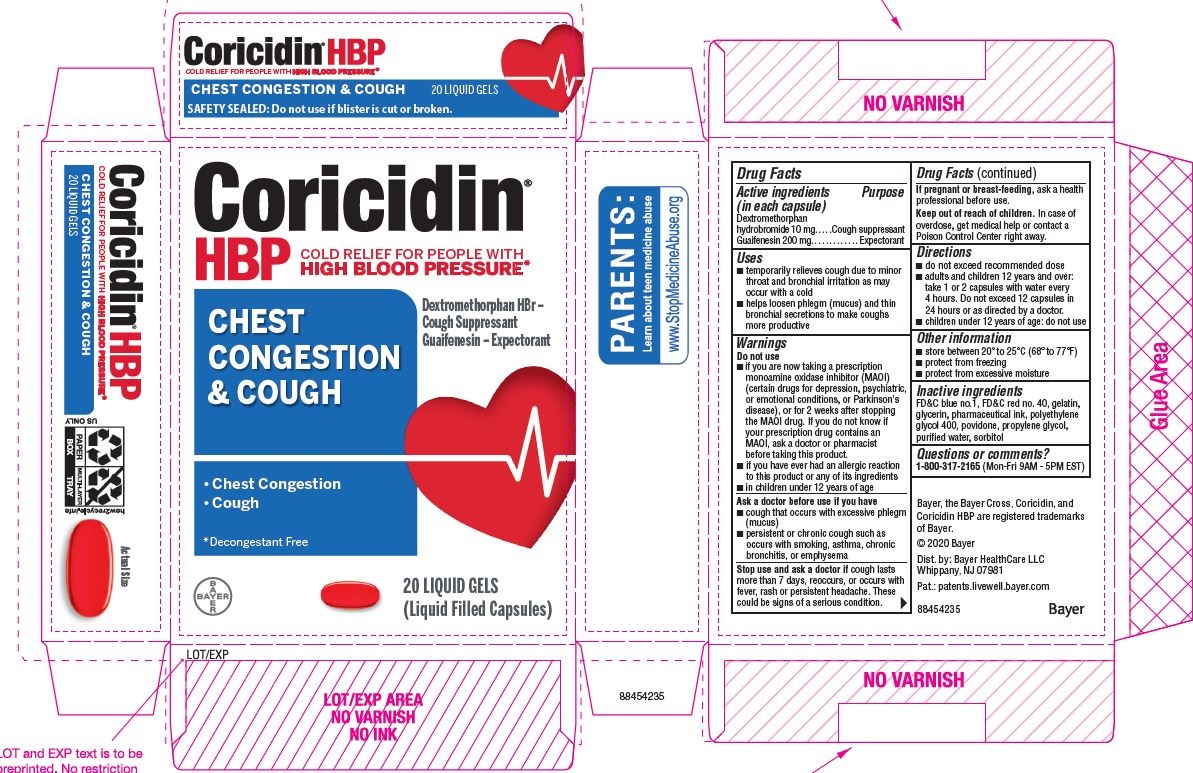
| Active ingredients (in each capsule) | Purpose |
| Dextromethorphan hydrobromide 10 mg | Cough suppressant |
| Guaifenesin 200 mg | Expectorant |
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- cough that occurs with excessive phlegm (mucus)
- persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- do not exceed recommended dose
- adults and children 12 years and over:
1 or 2 capsules every 4 hours, not more than
12 capsules in 24 hours - children under 12 years of age: ask a doctor
Other information
- store between 20° to 25°C (68° to 77°F)
- protect from freezing
- protect from excessive moisture
Inactive ingredients
FD&C red no. 40, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, shellac, sodium, sorbitol sorbitan solution, titanium dioxide.
Questions or comments?
Call 1-800-317-2165 between 8:00 AM and 5:00 PM Central Standard Time, Monday through Friday
PRINCIPAL DISPLAY PANEL - 2 x 10 Softgel Blister Pack Carton
Coricidin
®
HBP
COLD SYMPTOM RELIEF for people with
HIGH BLOOD PRESSURE
CHEST
CONGESTION
& COUGH
Relieves:
- Chest Congestion
- Cough
Guaifenesin - Expectorant,
Dextromethorphan hydrobromide -
cough suppressent
CONTROLS COUGH
FAST
Liqui-Gels ®
DECONGESTANT-FREE
American Heart Association
Health Information Enclosed
20 LIQUID GELS
(Liquid Filled Capsules)