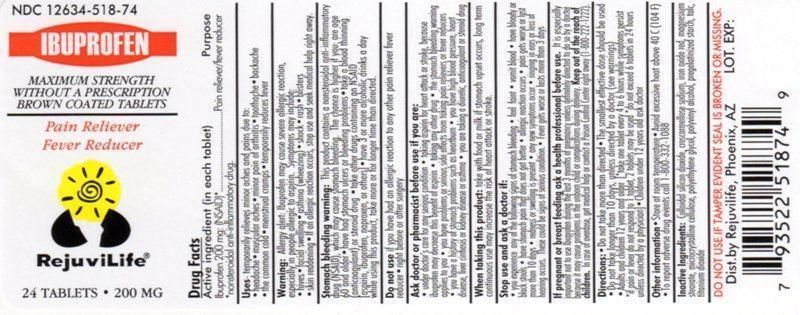
PURPOSE
Pain reliever / fever reducer
Use(s)
temporarily relieves minor aches and pain due to :
backache
headache
menstrual cramps
minor pain of arthritis
muscular aches
the common cold
toothache
temporarily reduces fever
WARNINGS
Allergy alerts: Ibuprofen may cause a severe allergy reaction, especially in people allergic to aspirin.
Symptoms may include:
- asthma (wheezing)
- blisters
- facial swelling
- hives
- rash
- shock
- skin reddening
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding. The chance is higher if you:
- are age 60 or older
- have bad stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drug containing prescription NSAID (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- the more or for a longer time than directed
DO NOT USE
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
ASK DOCTOR/PHARMACIST
Ask a doctor before use if you have
- problems or serious side effects from taking pain relievers or fever reducers
- stomach problems that last or come back, such as heartburn, upset stomach, or stomach pain
- ulcers
- bleeding problems
- high blood pressure
- heart or kidney disease
- taken a diuretics
- reached age 60 or older
Ask a doctor or pharmacist before use if you are
- taking any other drugs containg an NSAID (prescription or nonprescription)
- taking a blood thining (anticoagulant) or steriod drug
- under a doctor’s care for any serious condition
- taking aspirin for heart attacks or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
- long term continuous use may increase the risk of heart attack or stroke
Stop use and ask doctor if
- you feel faint, vomit blood, or have bloody or black stools
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- stomach pain or upset gets worse or lasts
- redness or swelling is present in painful area
- any new symptoms appear
PREGNACNCY OR BREAST FEEDING
Ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause a problems in the unborn child or complications during delivery.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a PoisonControlCenter right away. (1-800-222-1222)
INACTIVE INGREDIENTS
colloidal silicon dioxide, croscarmellose sodium, iron oxide red, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, pregelatinised starch, talc, titanium dioxide.
QUESTIONS
Directions
- do not take more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless directed by a docter
|
adults and children 12 years and older |
|
|
Children under 12 years |
|
Other information
store between 20-25 0c (68-77 0 F).
- do not use if seal under bottle cap imprinted with” SEALED for YOUR PROTECTION” is broken or missing
PRINCIPAL DISPLAY PANEL
Manufactured for:
Marksans Pharma Inc.
Lake Grove, NY11755, USA
Manufactured by:
Marksans Pharma Ltd.
Verna, Goa-403722, India
Repackaged and Distributed by:
Apotheca, Inc.
Phoenix, AZ 85006
Repackaged and Distributed for:
RejuviLife
Phoenix, AZ 85015
NDC 12634-518-74
IBUPROFEN
MAXIMUM STRENGTH
WITHOUT A PRESCRIPTION
BROWN COATED TABLETS
Pain Reliver
Fever Reducer
RejuviLife®
24 TABLETS 200MG