SANSERT- methysergide maleate tablet, coated
Novartis Pharmaceuticals Corporation
----------
Sansert®
T2000-69
89011201
Information for the physician
Sansert®
(methysergide maleate) tablets, USP
Rx only
Prescribing Information
WARNING
Retroperitoneal Fibrosis, Pleuropulmonary Fibrosis and Fibrotic Thickening of Cardiac Valves May Occur in Patients Receiving Long-term Methysergide Maleate Therapy. Therefore, This Preparation Must Be Reserved for Prophylaxis in Patients Whose Vascular Headaches Are Frequent and/or Severe and Uncontrollable and Who Are Under Close Medical Supervision.
(See also WARNINGS section)
DESCRIPTION
Sansert® (methysergide maleate) is a partially synthetic compound structurally related to lysergic acid butanolamide, well-known as methylergonovine in obstetrical practice as an oxytocic agent.
Chemically, methysergide maleate is designated as ergoline-8-carboxamide, 9,10-didehydro-N-[1-(hydroxymethyl)propyl]-1,6-dimethyl-, (8ß)-, (Z)-2-butenedioate (1:1) (salt).
Its structural formula is:
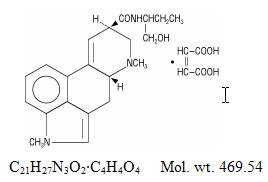
Methylation in the number 1 position of the ring structure enormously enhances the antagonism to serotonin which is present to a much lesser degree in the partially methylated compound (methylergonovine maleate) as well as profoundly altering other pharmacologic properties.
Active Ingredient: methysergide maleate, USP.
Inactive Ingredients: acacia, carnauba wax, colloidal silicon dioxide, FD&C Blue #1, FD&C Yellow #5, gelatin, lactose, malic acid, povidone, sodium benzoate, starch (corn), stearic acid, sucrose, synthetic black iron oxide, talc, and titanium dioxide.
ACTIONS
Sansert® (methysergide maleate) has been shown, in vitro and in vivo, to inhibit or block the effects of serotonin, a substance which may be involved in the mechanism of vascular headaches. Serotonin has been variously described as a central neurohumoral agent or chemical mediator, as a “headache substance” acting directly or indirectly to lower pain threshold (others in this category include tyramine; polypeptides, such as bradykinin; histamine; and acetylcholine), as an intrinsic “motor hormone” of the gastrointestinal tract, and as a “hormone” involved in connective tissue reparative processes. Suggestions have been made by investigators as to the mechanism whereby methysergide produces its clinical effects, but this has not been finally established.
INDICATIONS
For the prevention or reduction of intensity and frequency of vascular headaches in the following kinds of patients:
- Patients suffering from one or more severe vascular headaches per week.
- Patients suffering from vascular headaches that are uncontrollable or so severe that preventive therapy is indicated regardless of the frequency of the attack.
CONTRAINDICATIONS
Hypersensitivity to the drug or to tartrazine (FD&C Yellow #5) or any other components of the formulation, pregnancy, lactation, peripheral vascular disease, severe arteriosclerosis, severe hypertension, coronary artery disease, phlebitis or cellulitis of the lower limbs, pulmonary disease, collagen diseases or fibrotic processes, impaired liver or renal function, valvular heart disease, debilitated states and serious infections.
WARNINGS
With long-term, uninterrupted administration, retroperitoneal fibrosis or related conditions — pleuropulmonary fibrosis and cardiovascular disorders with murmurs or vascular bruits have been reported. Patients must be warned to report immediately the following symptoms and to discontinue the drug: cold, numb, and painful hands and feet; leg cramps on walking; any type of girdle, flank, or chest pain, shortness of breath, or any associated symptomatology. Should any of these symptoms develop, methysergide should be discontinued. Continuous administration should not exceed 6 months. There must be a drug-free interval of 3-4 weeks after each 6-month course of treatment. The dosage should be reduced gradually during the last 2-3 weeks of each treatment course to avoid “headache rebound.”
The drug is not recommended for use in children.
PRECAUTIONS
General
All patients receiving Sansert® (methysergide maleate) should remain under constant supervision of the physician and be examined regularly for the development of fibrotic or vascular complications. (See ADVERSE REACTIONS)
The manifestations of retroperitoneal fibrosis, pleuropulmonary fibrosis, and vascular shutdown have shown a high incidence of regression once Sansert® (methysergide maleate) is withdrawn. These facts should be borne in mind to avoid unnecessary surgical intervention. Cardiac murmurs, which may indicate endocardial fibrosis, have shown varying degrees of regression, with complete disappearance in some and persistence in others.
Sansert® (methysergide maleate) has been specifically designed for the prophylaxis of vascular headache and has no place in the management of the acute attack.
Sansert® (methysergide maleate) tablets contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Information for Patients
Sansert® (methysergide maleate) is intended for use as a preventive agent in the treatment of vascular headaches. It should not be used for acute migraine attacks. If, after a 3-week trial period, Sansert® (methysergide maleate) has not been effective in decreasing the frequency or intensity of headaches, it is unlikely that longer administration of Sansert® (methysergide maleate) will be beneficial.
Patients should be advised to report the following symptoms immediately and to discontinue the drug: cold, numb, and painful hands and feet; leg cramps on walking; any type of girdle, flank, or chest pain; shortness of breath; or any associated symptomatology. There must be a drug-free interval of 3-4 weeks after each 6-month course of treatment.
Sansert® (methysergide maleate) should be taken with meals. Weight gain may necessitate modification of diet.
Drug Interactions
Methysergide may reverse the analgesic activity of narcotic analgesics. Concurrent use with vasoconstrictor agents including ergot alkaloids, sumatriptan, and nicotine (e.g. smoking) may result in enhanced vasoconstriction.
Pregnancy Category X
Sansert® (methysergide maleate) is contraindicated in pregnancy due to its oxytocic actions.
Nursing Mothers
There are no specific studies on the use of Sansert® (methysergide maleate) in nursing mothers. Ergot alkaloids, in general, appear in mothers’ milk.
Sansert® (methysergide maleate) is a semi-synthetic compound structurally related to ergotamine, and thus it may appear in breast milk. Ergot alkaloids have been reported to cause nausea, vomiting, diarrhea and weakness in the nursing infant and suppression of prolactin secretion and lactation in the mother.
Because of the potential for serious adverse reactions in nursing infants from Sansert® (methysergide maleate), a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
Within the recommended dose levels, the following side effects have been reported:
1) Fibrotic Complications
Fibrotic changes have been observed in the retroperitoneal, pleuropulmonary, cardiac, and other tissues, either singly or, very rarely, in combination.
Retroperitoneal Fibrosis
This nonspecific fibrotic process is usually confined to the retroperitoneal connective tissue above the pelvic brim and may present clinically with one or more symptoms such as general malaise, fatigue, weight loss, backache, low grade fever (elevated sedimentation rate), urinary obstruction (girdle or flank pain, dysuria, polyuria, oliguria, elevated BUN), vascular insufficiency of the lower limbs (leg pain, Leriche syndrome, edema of legs, thrombophlebitis). The single most useful diagnostic procedure in suspected cases of retroperitoneal fibrosis is intravenous pyelography. Typical deviation and obstruction of one or both ureters may be observed.
Pleuropulmonary Complications
A similar nonspecific fibrotic process, limited to the pleural and immediately subjacent pulmonary tissues, usually presents clinically with dyspnea, tightness and pain in the chest, pleural friction rubs, and pleural effusion. These findings may be confirmed by chest X-ray.
2) Cardiovascular Complications
Encroachment of retroperitoneal fibrosis on the aorta, inferior vena cava and their common iliac branches may result in vascular insufficiency of the lower limbs, the presenting features of which are mentioned under Retroperitoneal Fibrosis.
Intrinsic vasoconstriction of large and small arteries, involving one or more vessels or merely a segment of a vessel, may occur at any stage of therapy. Depending on the vessel involved, this complication may present with chest pain, abdominal pain, or cold, numb, painful extremities with or without paresthesias and diminished or absent pulses. Progression to ischemic tissue damage has rarely been reported. Prompt withdrawal of the drug at the first signs of impaired circulation is recommended (see WARNINGS) to obviate such effects.
Postural hypotension and tachycardia have also been observed.
3) Gastrointestinal Symptoms
Nausea, vomiting, diarrhea, heartburn, abdominal pain. These effects tend to appear early and can frequently be obviated by gradual introduction of the medication and by administration of the drug with meals. Constipation and elevation of gastric HCl have also been reported.
4) CNS Symptoms
Seizure, insomnia, drowsiness, mild euphoria, dizziness, ataxia, lightheadedness, hyperesthesia, unworldly feelings (described variously as “dissociation”, “hallucinatory experiences”, etc.). Some of these symptoms may be associated with vascular headaches, per se, and may, therefore, be unrelated to the drug.
5) Dermatological Manifestations
Facial flush, telangiectasia, and nonspecific rashes have rarely been reported. Increased hair loss may occur, but in many instances the tendency has abated despite continued therapy.
OVERDOSAGE
Few cases of acute Sansert® (methysergide maleate) intoxication have been reported. The possible symptom complex is therefore not fully known. The following symptoms are based on these few case reports. Euphoria, hyperactivity, tachycardia, dilated pupils, and dizziness have been reported in a child with a dose of 20-24 mg of Sansert® (methysergide maleate). In adults, peripheral vasospasm, with diminished or absent pulses, coldness, mottling and cyanosis, has been observed at a dose of 200 mg. Ischemic tissue damage has not been reported in acute overdosage with Sansert® (methysergide maleate).
Treatment consists of removal of the offending drug by induction of emesis, or gastric lavage in the case of very recent intake, repeat dose administration of activated charcoal and catharsis. There is no evidence that forced diuresis accelerates the elimination of Sansert® (methysergide maleate). However, I.V. fluids may be given as a general supportive measure.
Treatment of peripheral vasospasm should consist of warmth, but not heat, and protection of the ischemic limbs. In reported cases of Sansert® (methysergide maleate) overdosage, the use of vasodilators has not been necessary. However, if vasospasm is persistent, severe, or if there is evidence of impending ischemic tissue damage, these agents may be beneficial. Careful nursing care is recommended in order to prevent tissue damage.
Up-to-date information about the treatment of overdose can often be obtained from a certified Regional Poison Control Center. Telephone numbers of certified Regional Poison Control Centers are listed in the Physicians’ Desk Reference®.*
DOSAGE AND ADMINISTRATION
Usual adult dose 4-8 mg daily. Tablets to be given with meals.
Note: There must be a medication-free interval of 3-4 weeks after every 6-month course of treatment. (See WARNINGS)
No pediatric dosage has been established.
If, after a 3-week trial period, efficacy has not been demonstrated, longer administration of Sansert® (methysergide maleate) is unlikely to be of benefit.
ANIMAL PHARMACOLOGY AND TOXICOLOGY
Serotonin Antagonism
Considering structure/effect relationships, it has been demonstrated that methylation of the indole nitrogen in the lysergic acid ring of the alkanolamides fundamentally alters their pharmacologic behavior and is associated with inhibition or blockade of a great variety of serotonin-induced effects:
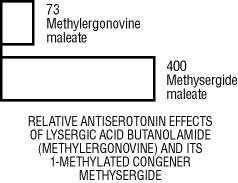
| 1. | Methysergide maleate is 6 times more active than methylergonovine maleate in antagonizing the effect of serotonin on the rat uterus in vitro. |
 |
|
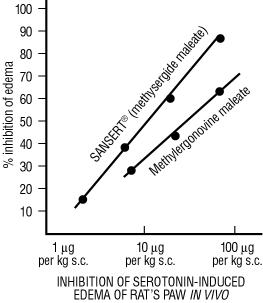
| 2. | Greater inhibition of the serotonin-induced edema in the rat’s paw is revealed by the ED50 of 12.9 µg/kg for methysergide maleate as against 37.4 µg/kg for methylergonovine maleate (see following graph). |
 |
|
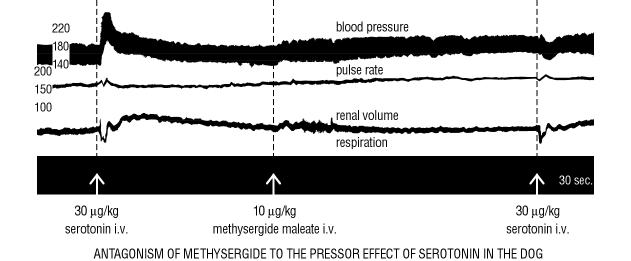
| 3. | The more complex effects of serotonin on the cardiovascular system are equally subject to inhibition by methysergide maleate as is evident from the subsequent record of various circulatory parameters in the dog before and after administration of 10 µg/kg. |
 |
| Acute Toxicity | |||||
| LD50 in mg/kg | |||||
| Mice | Rabbits | Rats | |||
| Compound | i.v. | oral | i.v. | i.v. | oral |
| Methysergide maleate | 185 | 581 | 28 | 125 | 2100 |
| methylergonovine maleate | 85 | 187 | 2.6 | 23 | 93 |
| Chronic Toxicity | ||
| Rats | ||
| Daily Oral Doses
mg/kg | No. of Weeks
Animals Tested | Mortality |
| 5 | 50 | 4/16 |
| 20 | 50 | 2/16 |
| 50 | 50 | 2/16 |
| 150 | 17 | 8/30 |
| 450 | 17 | 17/30 |
| Control | 50 | 1/16 |
Dogs
Oral administration of 1, 2, and 5 mg/kg/day of methysergide maleate failed to produce any major signs of toxicity over a period of 6 months.
*Trademark of Medical Economics Company, Inc.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
REV: NOVEMBER 2000 T2000-69
| SANSERT
methysergide maleate tablet, coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |
