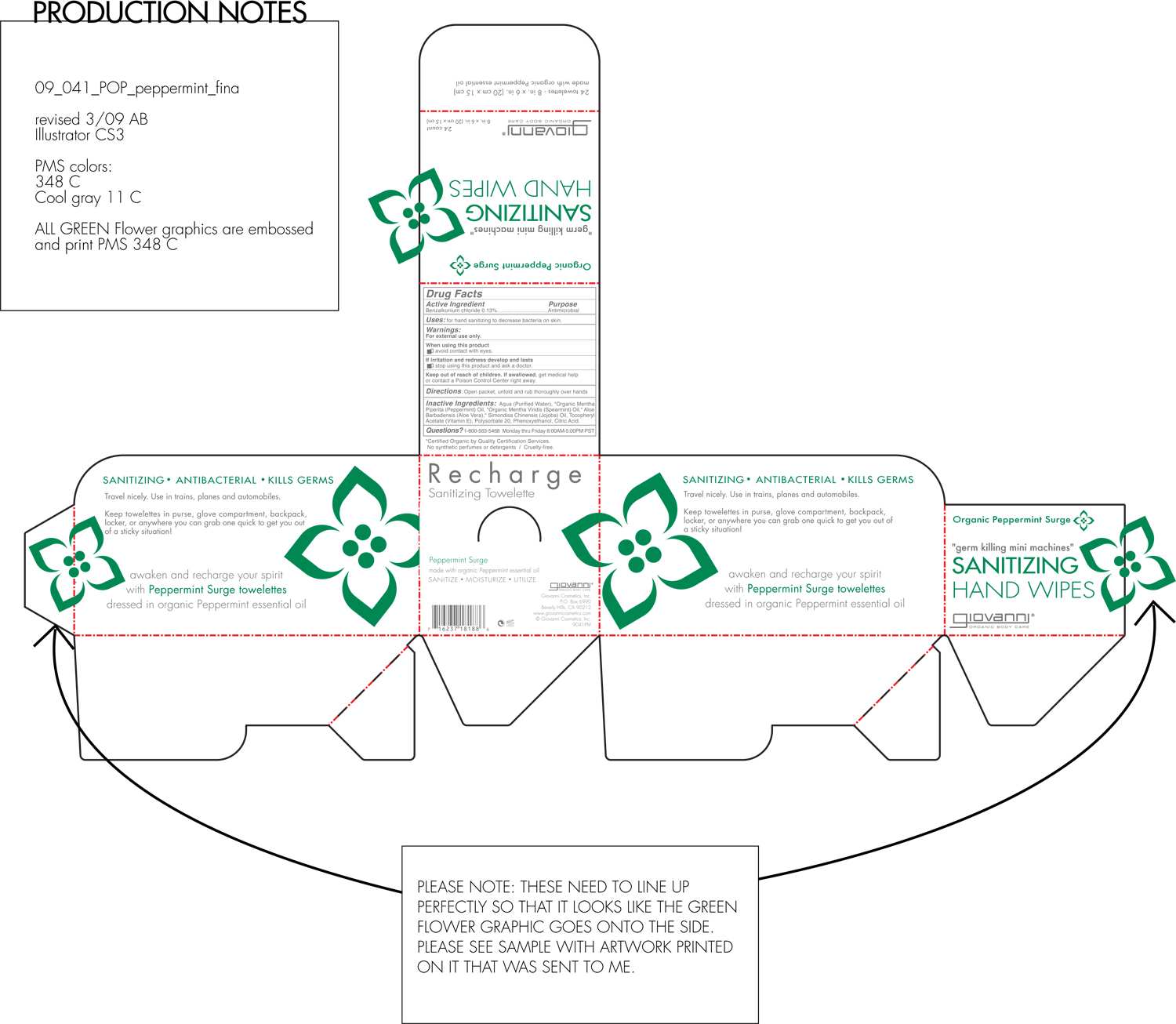
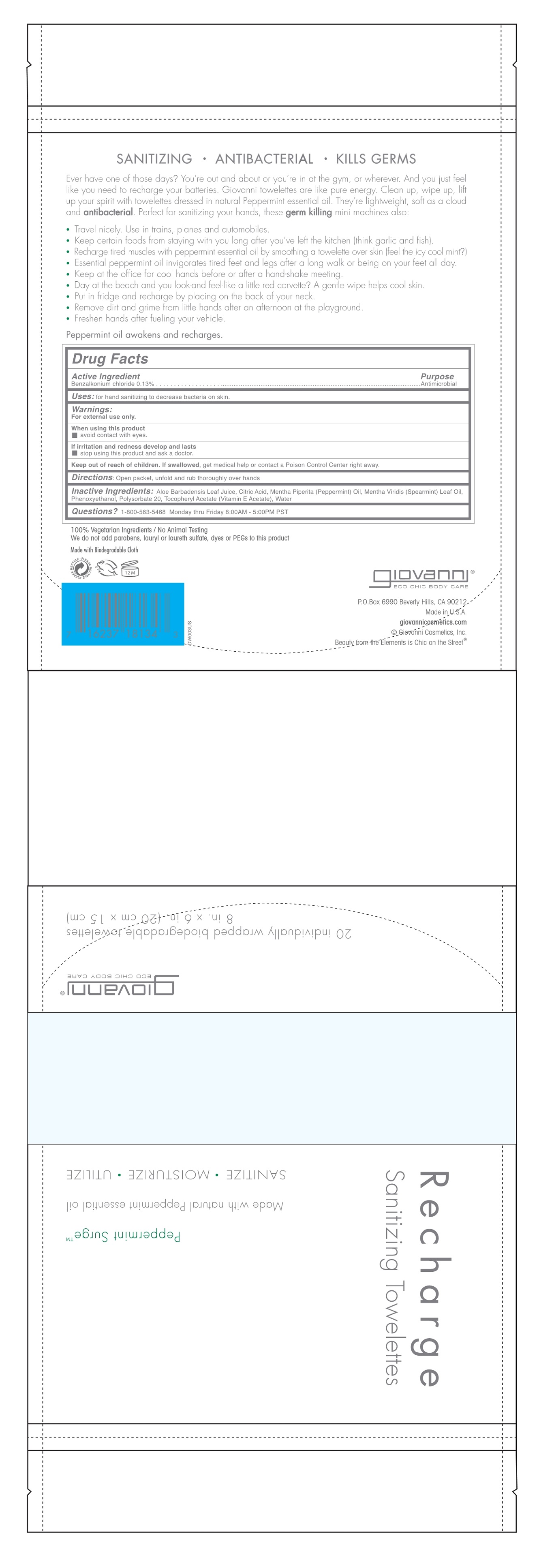
RECHARGE SANITIZING TOWLETTE ORGANIC PEPPERMINT SURGE- benzalkonium chloride liquid
GIOVANNI COSMETICS INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Benzalkonium Chloride 0.13%
Uses
For hand sanitizing to decrease bacteria on skin.
Questions
Questions? 1-800-563-5458 Monday thru Friday 8:00 AM - 5:00 PM PST
Keep Out Of Reach Of Children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Open packet, unfold and rub thoroughly over hands.
Warnings
For external use only.
When using this product avoid contact with eyes.
If irritation and redness develop and lasts, stop using this product and ask a doctor.
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Citric Acid, Mentha Piperita (Peppermint) Oil, Mentha Virdis (Spearmint) Leaf Oil, Phenoxyethanol, Polysorbate 20, Tocopheryl Acetate (Vitamin E Acetate), Water
Uses
For hand sanitizing to decrease bacteria on the skin.
Recharge Sanitizing Towelette
Peppermint Surge
Made with organic peppermint essential oil