DIRECTIONS
Tilt head forward, spray 2-3 times in each nostril directing spray upward and outward toward ear and away from center of nose. Blow nose. For best results, see package insert for the Vanderpool Technique™ for a more thorough nasal cleansing procedure or go to the NasalAsalt® web site for a video demonstration.
WARNINGS
PRECAUTIONS
Do not use with children under 6 years of age except as directed by a physician. Use of this dispenser by more than one person may spread infection.
Distributed by:
Nasal and Sinus Health, Inc., 16 Spring Hill Court, Bluffton, SC 29910. You may report serious side effects to this address.
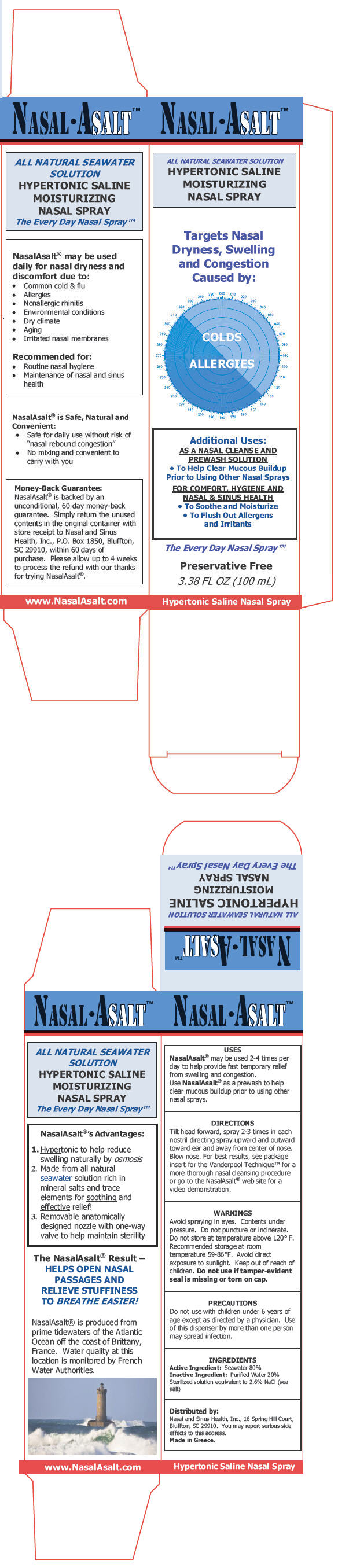
PRINCIPAL DISPLAY PANEL - 100 mL Canister Carton
NASAL•ASALT™
ALL NATURAL SEAWATER SOLUTION
HYPERTONIC SALINE
MOISTURIZING
NASAL SPRAY
Targets Nasal
Dryness, Swelling
and Congestion
Caused by:
COLDS
ALLERGIES
Additional Uses:
AS A NASAL CLEANSE AND
PREWASH SOLUTION
-
To Help Clear Mucous Buildup
Prior to Using Other Nasal Sprays
FOR COMFORT, HYGIENE AND
NASAL & SINUS HEALTH
- To Soothe and Moisturize
-
To Flush Out Allergens
and Irritants
The Every Day Nasal Spray™
Preservative Free
3.38 FL OZ (100 mL)
Hypertonic Saline Nasal Spray