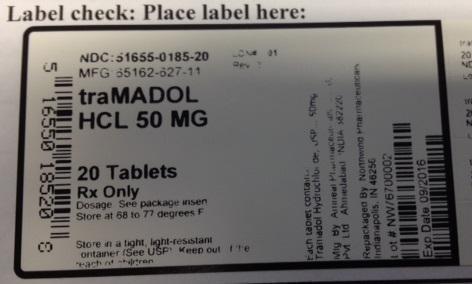
NDC: 51655-0185-20
MFG: 65162-627-11
traMADOL HCL 50 MG
20 TABLETS RX ONLY
DOSAGE: SEE PACKAGE INSERT
STORE AT 68 TO 77 DEGREES F
STORE IN A TIGHT, LIGHT-RESISTANT CONTAINER. (SEE USP) KEEP OUT OF REACH OF CHILDREN
EACH TABLET CONTAINS TRAMADOL HYDROCHLORIDE USP 50 MG
MFG BY: AMNEAL PHARMACEUTICALS PVT LTD AHMEDABAD INDIA 382220
REPACKAGED BY NORTHWIND PHARMACEUTICALS INDIANAPOLIS, IN 46256
LOT# NW76700002
EXP DATE: 09/2016