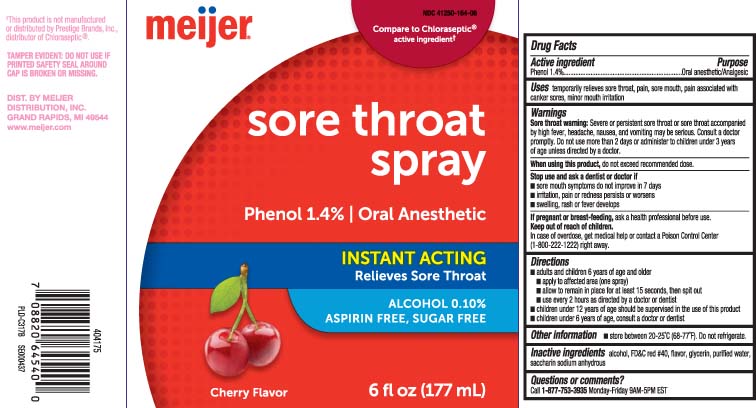
Uses
temporarily relieves sore throat pain, sore mouth, pain associated with canker sores, minor mouth irritation
Warnings
Sore throat warning: Severe or persistent sore throat or sore throat that occurs with high fever, headache, nausea, and vomiting may be serious. Ask a doctor right away. Do not use more than 2 days or give to children under 3 years of age.
Directions
- adults and children 6 years of age and older
- apply to affected area (one spray)
- allow to remain in place for at least 15 seconds, then spit out
- use every 2 hours as directed by a doctor or dentist
- children under 12 years of age should be supervised in the use of this product
- children under 6 years of age, consult a doctor or dentist
Principal Display Panel
Compare to Chloraseptic® active ingredient†
Sore Throat Spray
Phenol 1.4% | Oral Anesthetic
INSTANT ACTING
Relieves Sore Throat
ALCOHOL 0.10%
ASPIRIN FREE, SUGAR FREE
Cherry Flavor
FL OZ (mL)
†This product is not manufactured or distributed by Prestige Brands, Inc., distributor of Chloraseptic®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL AROUND CAP IS BROKEN OR MISSING.
DIST. BY MEIJER
DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544