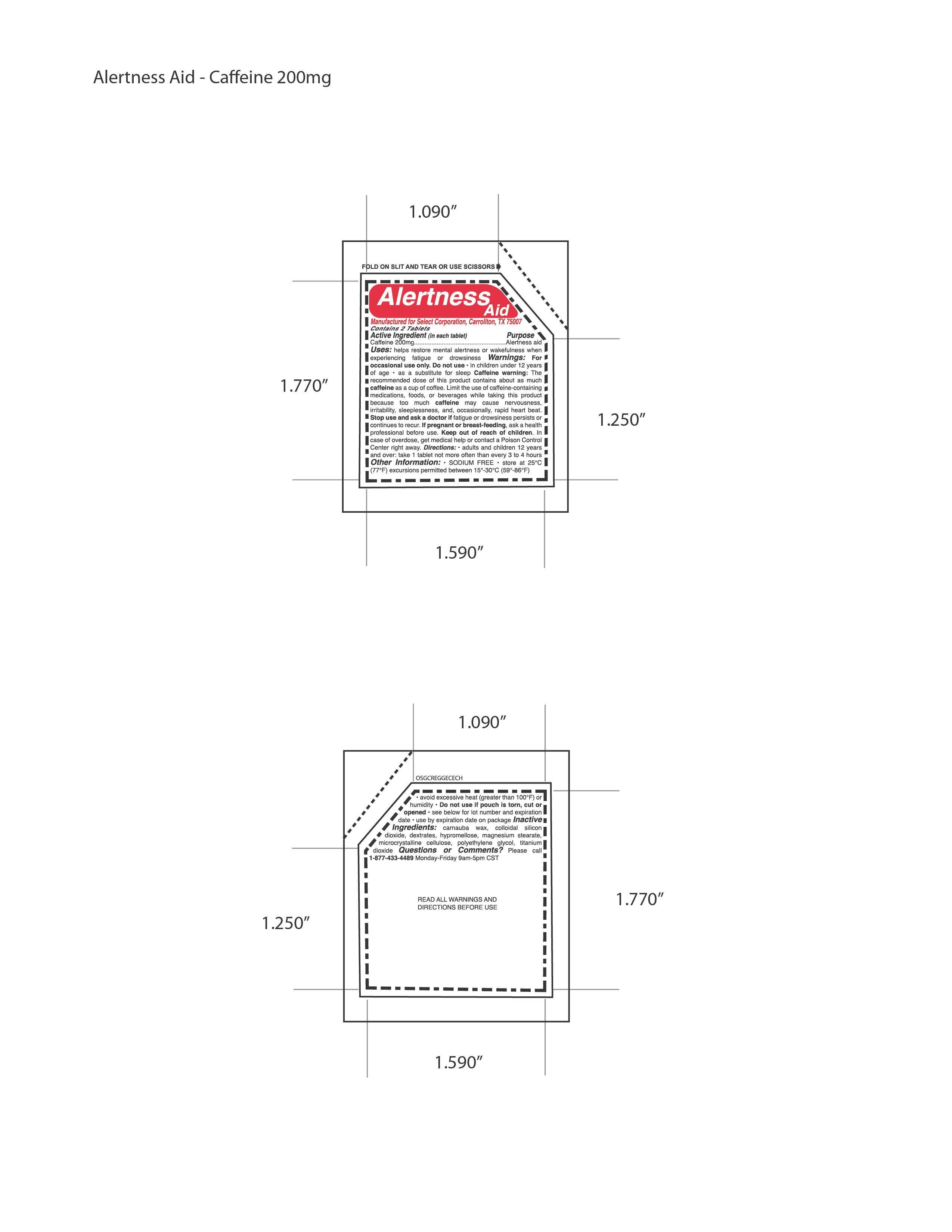
Directions: • adults and children 12 years
and over: take 1 tablet not more often than every 3 to 4 hours
and over: take 1 tablet not more often than every 3 to 4 hours
Warnings: For
occasional use only. Do not use • in children under 12 years
of age • as a substitute for sleep Caffeine warning: The
recommended dose of this product contains about as much
caffeine as a cup of coffee. Limit the use of caffeine-containing
medications, foods, or beverages while taking this product
because too much caffeine may cause nervousness,
irritability, sleeplessness, and, occasionally, rapid heart beat.
Stop use and ask a doctor if fatigue or drowsiness persists or
continues to recur.
occasional use only. Do not use • in children under 12 years
of age • as a substitute for sleep Caffeine warning: The
recommended dose of this product contains about as much
caffeine as a cup of coffee. Limit the use of caffeine-containing
medications, foods, or beverages while taking this product
because too much caffeine may cause nervousness,
irritability, sleeplessness, and, occasionally, rapid heart beat.
Stop use and ask a doctor if fatigue or drowsiness persists or
continues to recur.