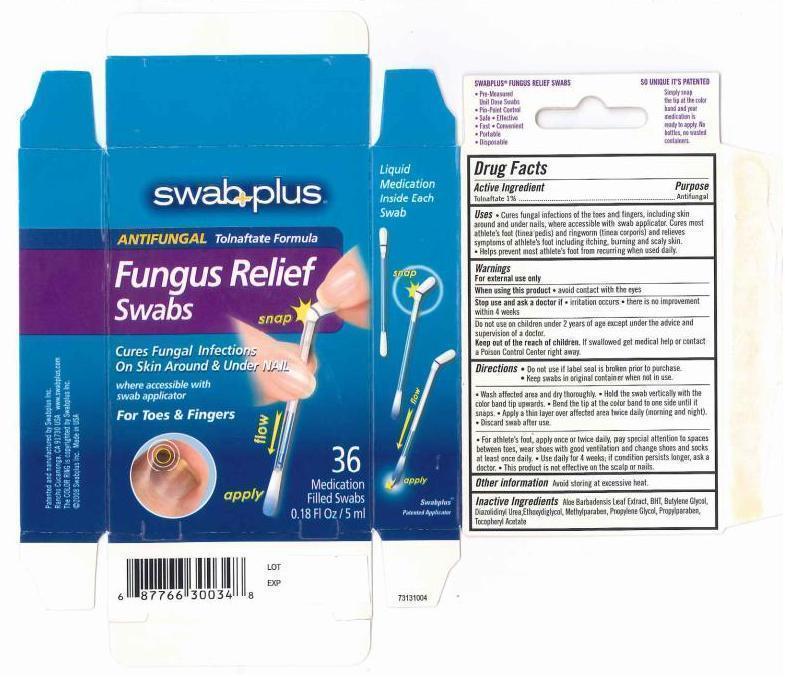
Uses
- Cures fungal infections of the toes and fingers, including skin around and under nails, where accessible with swab applicator. Cures most athlete's foot (tinea pedis) and ringworm (tinea corporis) and relieves symptoms of athlete's foot including itching, burning and scaly skin.
- Help prevent most athlete's foot from recuring when uses daily.
Warings
For external use only.
When using this product avoid contact with eyes.
Stop use and ask a doctor if irritation occurs. there is no improvement within 4 weeks.
Do not use on children under 2 years of age except under the advice and supervision of a doctor.
Keep out of the reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- Do not use if label seal is broken prior to purchase.
- Keep swabs in original container when not in use.
- wash affected area and dry thoroughly.
- Hold the swab vertically with the color band tip upwards.
- Bend the tip at the color band to one side until it snaps.
- Apply a thin layer over affected area twice daily (morning and night)
- Discard swab after use.
- For athlete's foot, apply once or twice daily, pay special attention to spaces between toes, wear shoes with good ventilation and change shoes and socks at least once daily.
- Use daily for 4 weeks; if condition persists longer, ask a doctor.
- This product is not effective on the scalp or nails.