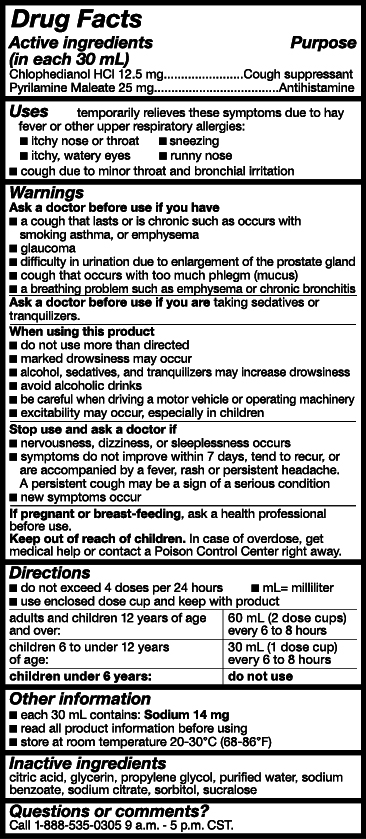
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- itchy nose or throat
- sneezing
- itchy, watery eyes
- runny nose
- cough due to minor throat and bronchial irritation
Warnings
Ask doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
- glaucoma
- difficulty in urination due to the enlargment of the prostate gland
- cough that occurs with too much phlegm(mucus)
- a breathing problem such as emphysema or chronic bronchitis
When using this product
- do not use more than directed
- marked drowsiniess may occur
- alcohol, secatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especiially in children
Stop use and ask a doctor if
- Nervousness, dizziness, or sleeplessness occurs
- symptoms do not improve within 7 days, tend to recur, or are accompanied by a fever, rash or persisten headace. A persisten cough may be a sign of a serious condition.
- new symptoms occur
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Do not exceed 4 doses per 24-hours.
- Use enclosed dose cup and keep with product.
- mL = mililiter
| Adults and children 12 years of age and over: | 60 mL (2 dose cups) every 6 to 8 hours |
| Children 6 to under 12 years of age: | 30 mL (1 dose cup) every 6 to 8 hours |
| Children under 6 years of age: | Do not use. |
Other Information
- each 30 mL contains: Sodium 14 mg
- read all product information before using
- store at room temperature 68-86°F (20-30°C
Inactive ingredients
citric acid, glycerin,propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol, sucralose
Questions or comments?
Call 1-888-535-0305 9 a.m. - 5 p.m. CST.
Distributed by:
GM Pharmaceuticals, Inc.
Fort Worth, TX 76118
KEEP LEAFLET AFTER OPENING
TexaClear® Kids Allergy + Cough
NDC 58809-925-08
8 fl. oz. (237 mL)
Chlophedianol HCl – Cough Suppressant
Pyrilamine Maleate – Antihistamine
Tamper evident: do not use if foil seal under cap is broken on missing
- Gluten Free
- Dye Free
- Sugar Free
- Alcohol Free
- Acetaminophen Free
Ages 6+
Dose every 6 to 8 hours
- Sneezing
- Runny Nose
- Watery Eyes
- Itchy Nose & Throat
- Cough
Distributed by:
GM Pharmaceuticals, Inc. Fort Worth, TX 76118