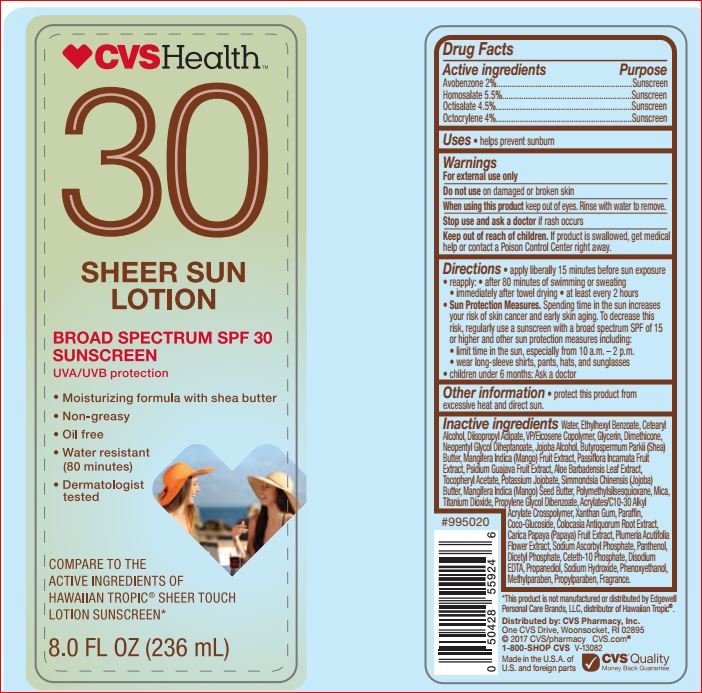
Active ingredients Purpose
Avobenzone 2.00%..........................Sunscreen
Homosalate 5.50%...........................Sunscreen
Octisalate 4.50%..............................Sunscreen
Octocrylene 4.00%...........................Sunscreen
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away
Directions
• Apply liberally 15 minutes before sun exposure
• Use a water resistant sunscreen if swimming
or sweating
• Reapply at least every 2 hours
• Children under 6 months: Ask a doctor
• Sun Protection Measures. Spending time in
the sun increases your risk of skin cancer and
early skin aging. To decrease this risk, regularly
use a sunscreen with a broad spectrum SPF
of 15 or higher and other sun protection
measures including: • limit time in the sun,
especially from 10 a.m. – 2 p.m. • wear
long-sleeve shirts, pants, hats, and sunglasses
Inactive ingredients
Water
Ethylhexyl Benzoate
Cetearyl Alcohol
Diisopropyl Adipate
VP/Eicosene Copolymer
Glycerin
Dimethicone
Neopentyl Glycol Diheptanoate
Jojoba Alcohol
Butyrospermum Parkii (Shea) Butter
Mangifera Indica (Mango) Fruit Extract
Passiflora Incarnata Fruit Extract
Psidium Guajava Fruit Extract
Aloe Barbadensis Leaf Extract
Tocopheryl Acetate
Potassium Jojobate
Simmondsia Chinensis (Jojoba) Butter
Mangifera Indica (Mango) Seed Butter
Polymethylsilsesquioxane
Mica
Titanium Dioxide
Propylene Glycol Dibenzoate
Acrylates/C10-30 Alkyl Acrylate Crosspolymer
Xanthan Gum
Paraffin
Coco-Glucoside
Colocasia Antiquorum Root Extract
Carica Papaya (Papaya) Fruit Extract
Plumeria Acutifolia Flower Extract
Sodium Ascorbyl Phosphate
Panthenol
Dicetyl Phosphate
Ceteth-10 Phosphate
Disodium EDTA
Propanediol
Sodium Hydroxide
Phenoxyethanol
Methylparaben
Propylparaben
Fragrance