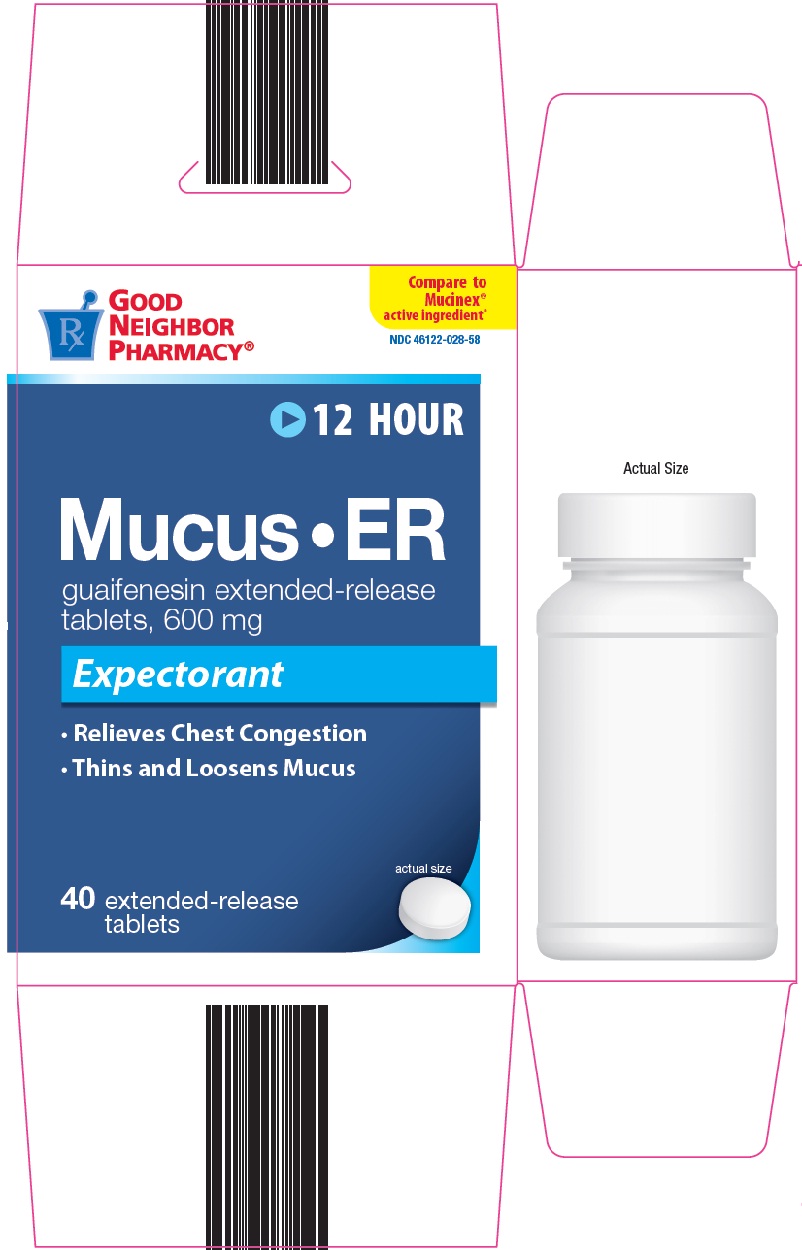
GOOD NEIGHBOR PHARMACY MUCUS ER- guaifenesin tablet, extended release
Amerisource Bergen
----------
Amerisource Bergen Mucus•ER Drug Facts
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
Directions
- •
- do not crush, chew, or break tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for the timing of meals
- •
- adults and children 12 years of age and over: one or two tablets every 12 hours. Do not exceed 4 tablets in 24 hours.
- •
- children under 12 years of age: do not use
Other information
- •
- do not use if printed seal under cap is broken or missing
- •
- store between 20-25°C (68-77°F)
| GOOD NEIGHBOR PHARMACY MUCUS ER
guaifenesin tablet, extended release |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Amerisource Bergen (007914906) |
Revised: 11/2017
Document Id: 34670ba9-a3de-44d8-b212-819e470f09dc
Set id: 3aa8a59f-06dd-4f1f-85af-da7eaef6883d
Version: 4
Effective Time: 20171125
Amerisource Bergen