Belladonna HPUS 6c, 12c, 18c
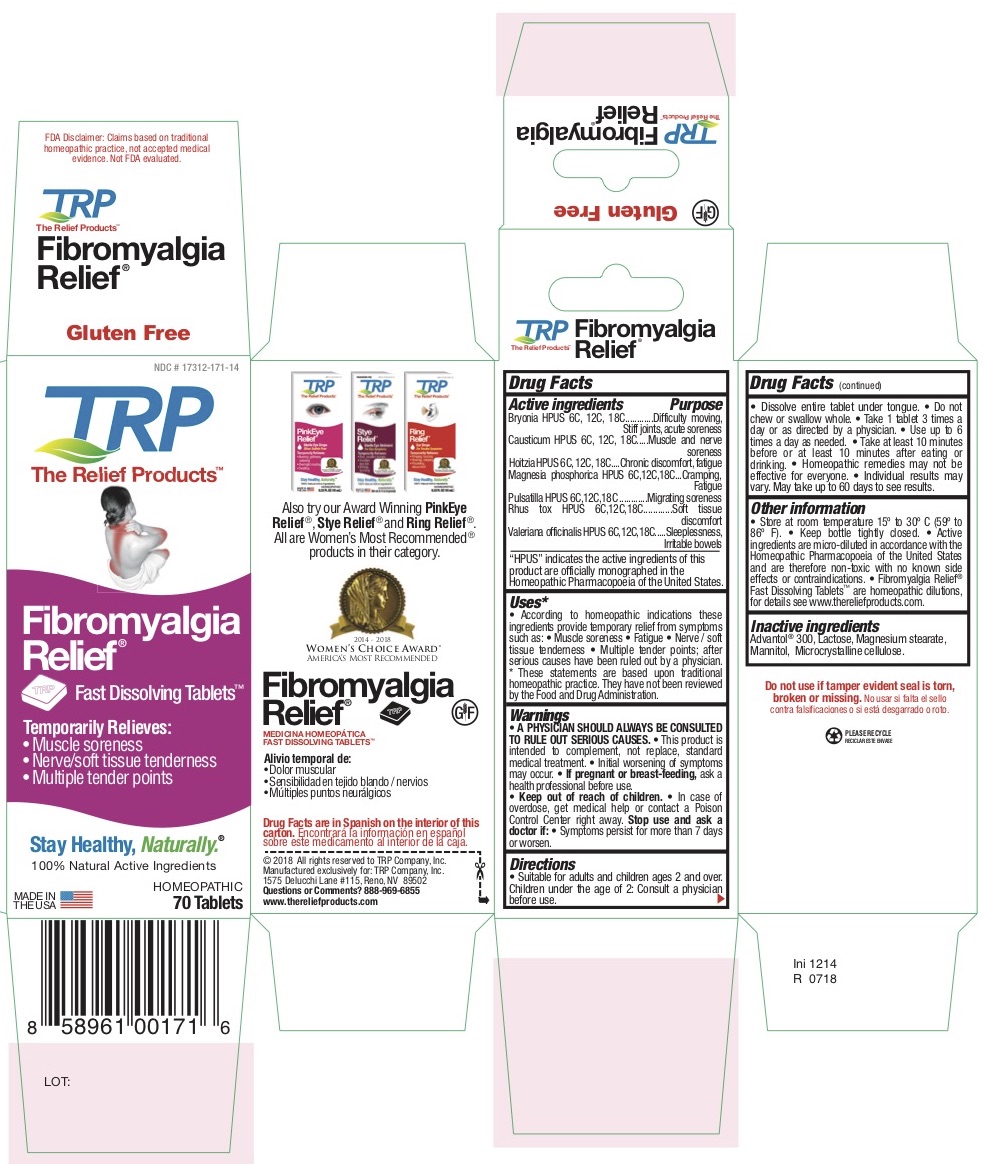
Bryonia HPUS 6c, 12c, 18c
Causticum HPUS 6c, 12c, 18c
Magnesia Phosphorica HPUS 6c,12c,18c
Nux Vomica HPUS 6c,12c,18c
Pulsatilla HPUS 6c,12c,18c
Rhus Tox HPUS 6c,12c,18c
HPUS indicates the active ingredients are in the Homeopathic Pharmacopoeia of the United States.
Belladonna HPUS - Chronic discomfort, Fatigue
Bryonia HPUS - Difficulty moving, Stiff joints, Acute soreness
Causticum HPUS - Muscle and nerve soreness
Magnesia Phosphorica HPUS - Cramping, Fatigue
Nux Vomica HPUS - Sleeplessness, Irritable bowels
Pulsatilla HPUS - Migrating soreness
Rhus Tox HPUS - Soft tissue discomfort
Uses
According to homeopathic indications these ingredients provide temporary relief from symptoms of Fibromyalgia such as: • Muscle soreness • Fatigue • Nerve / Soft tissue tenderness • Multiple tender points after diagnosis by a physician.
* These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
Directions
- Suitable for adults and children 12 years and above.
- Dissolve entire tablet under tongue.
- Do not chew or swallow whole.
- Take 1 tablet 3 times a day or as directed by a physician.
- Use up to 6 times a day as needed.
- Take at least 10 minutes before or at least 10 minutes after eating or drinking.
- Children under the age of 12: consult a physician before use.
Other information
There are no known contraindications.
- Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side cool dark location. Temporary Fibromyalgia Pain and Discomfort Relief® Fast Dissolving TabletsTM are homeopathic dilutions, for details see www.thereliefproducts.com.
Inactive Ingredients
Advantol® 300, Lactose, Magnesium Stearate, Mannitol, Microcrystalline Cellulose.
Stop use
Stop use and ask a doctor if:
Symptoms persist for more than 7 days or worsen, if new symptoms occur or if redness or swelling is present because these could be a sign of a serious illness.
Warning
USE ONLY AFTER DIAGNOSIS BY A PHYSICIAN.
- This product is intended to complement, not replace, standard medical treatment.
- Initial worsening of symptoms may occur.
- A physician should always be consulted regarding Fibromyalgia to rule out serious causes.
- In case of overdose, get medical help or contact a Poison Control Center right away.