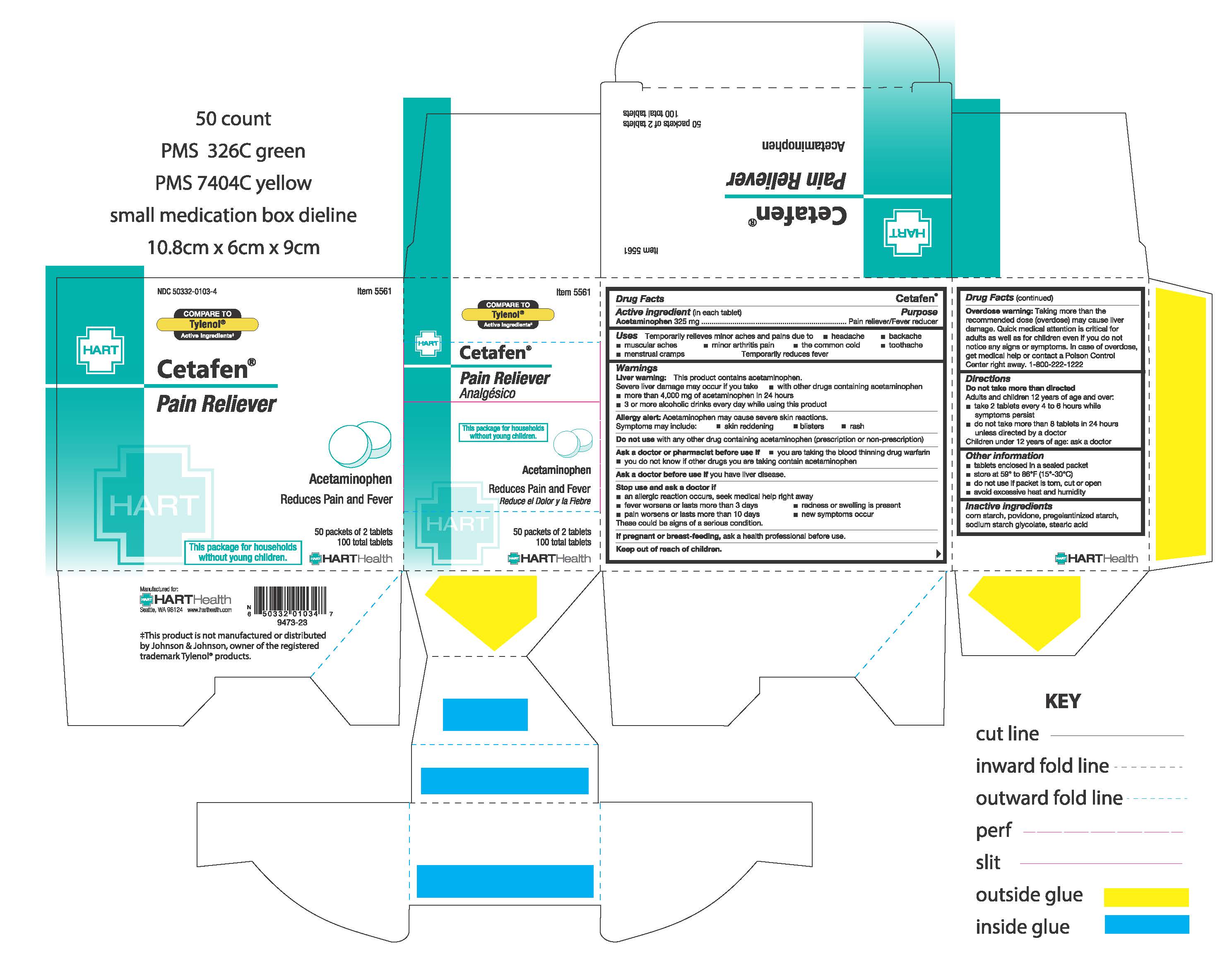
Uses:
Temporarily relieves minor aches and pains due to:
- headache
- muscular aches
- backache
- minor arthritis pain
- the common cold
- toothache
- menstrual cramps
Temporarily reduces fever
Warnings:
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- rash
- blisters
Ask a doctor or pharmacist before use if:
- you are taking the blood thinning drug warfarin
- you do not know if other drugs you are taking contain acetaminophen
Stop use and ask a doctor if:
- an allergic reaction occurs, seek medical help right away
- new symptoms occur
- redness or swelling is present
- fever worsens or lasts for more than 3 days
- pain worsens or lasts for more than 10 days
These could be signs of a serious condition
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms. In case of overdose, get medical help or contact Poison Control Center right away. 1-800-222-1222
Directions:
Do not take more than directed.
Adults and children 12 year of age and over:
- take 2 tables every 4 to 6 hours while symptoms persist
- do not take more than 8 tablets in 24 hours unless directed by a doctor
Children under 12 years of age: ask a doctor
Other information:
- tablets enclosed in a sealed packet
- do not use if packet is torn, cut or open
- store at 59° to 86°F (15°-30°C)
- avoid excessive heat and humidity