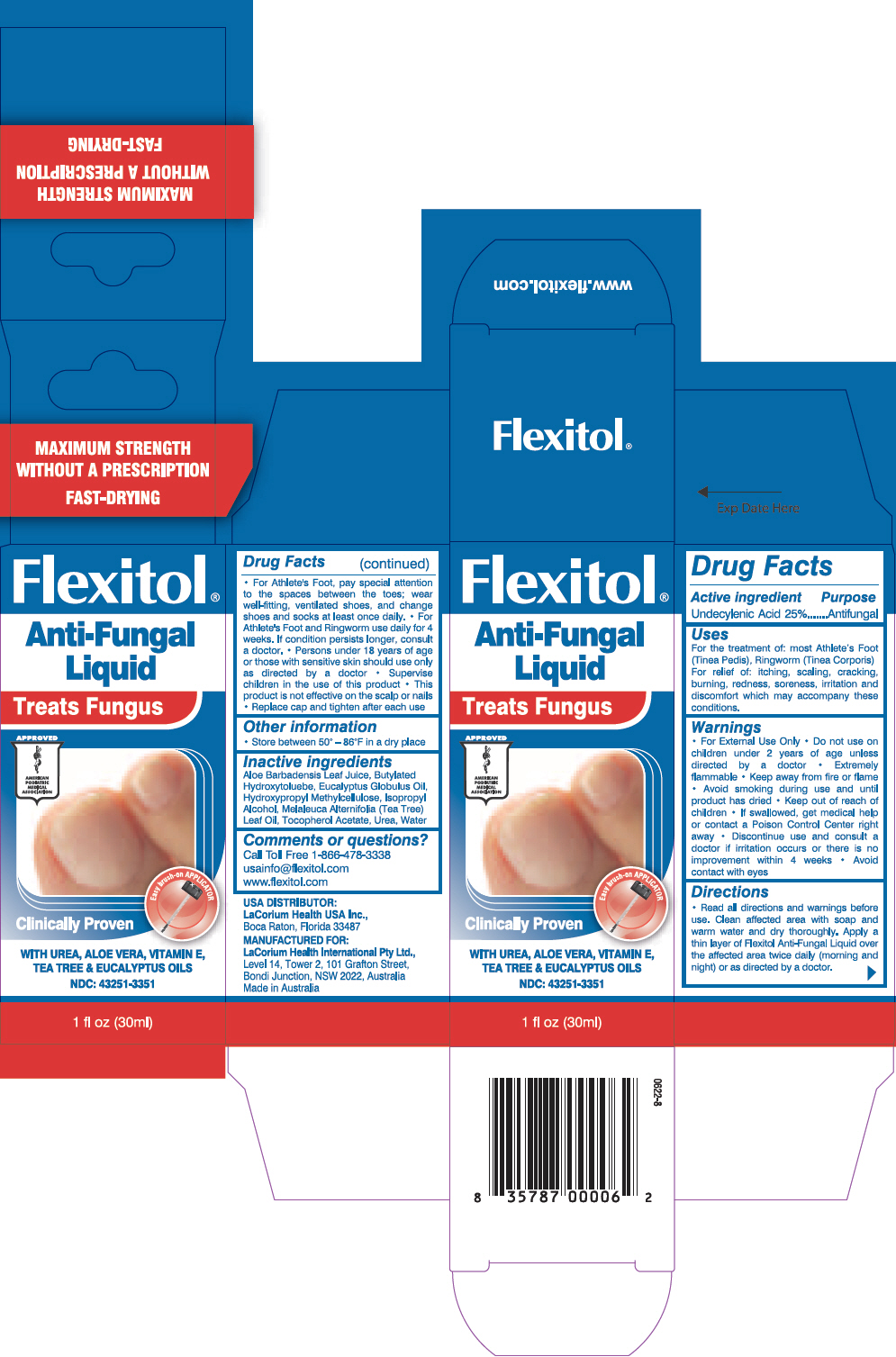
FLEXITOL ANTI FUNGAL- undecylenic acid liquid
LaCorium Health International Pty Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Undecylenic Acid 25%
Uses
For the treatment of: most Athlete's Foot (Tinea Pedis), Ringworm (Tinea Corporis). For relief of: itching, scaling, cracking, burning, redness, soreness, irritation and discomfort which may accompany these conditions.
Warnings
- Do not use on children under 2 years of age unless directed by a doctor
- Extremely flammable
- Keep away from fire or flame
- Avoid smoking during use and until product has dried
- Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
- Discontinue use and consult a doctor if irritation occurs or there is no improvement within 4 weeks
Directions
- Read all directions and warnings before use
- Clean affected area with soap and warm water and dry thoroughly
- Apply a thin layer of Flexitol Anti-Fungal Liquid over the affected area twice daily (morning and night) or as directed by a doctor
- For Athlete's Foot, pay special attention to the spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
- For Athlete's Foot and Ringworm use daily for 4 weeks. If condition persists longer, consult a doctor.
- Persons under 18 years of age or those with sensitive skin should use only as directed by a doctor
- Supervise children in the use of this product
- This product is not effective on the scalp or nails
- Replace cap and tighten after each use
Other information
Store between 50° – 86°F in a dry place
Inactive ingredients
Aloe Barbadensis Leaf Juice, Butylated Hydroxytoluebe, Eucalyptus Globulus Oil, Hydroxypropyl Methylcellulose, Isopropyl Alcohol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Tocopherol Acetate, Urea, Water
Comments or questions?
Call Toll Free 1-866-478-3338
usainfo@flexitol.com
www.flexitol.com
PRINCIPAL DISPLAY PANEL - 30ml Bottle Carton
Flexitol®
Anti-Fungal
Liquid
Treats Fungus
APPROVED
AMERICAN
PODIATRIC
MEDICAL
ASSOCIATION
Clinically Proven
Easy brush-on APPLICATOR
WITH UREA, ALOE VERA, VITAMIN E,
TEA TREE & EUCALYPTUS OILS
NDC: 43251-3351
1 fl oz (30ml)