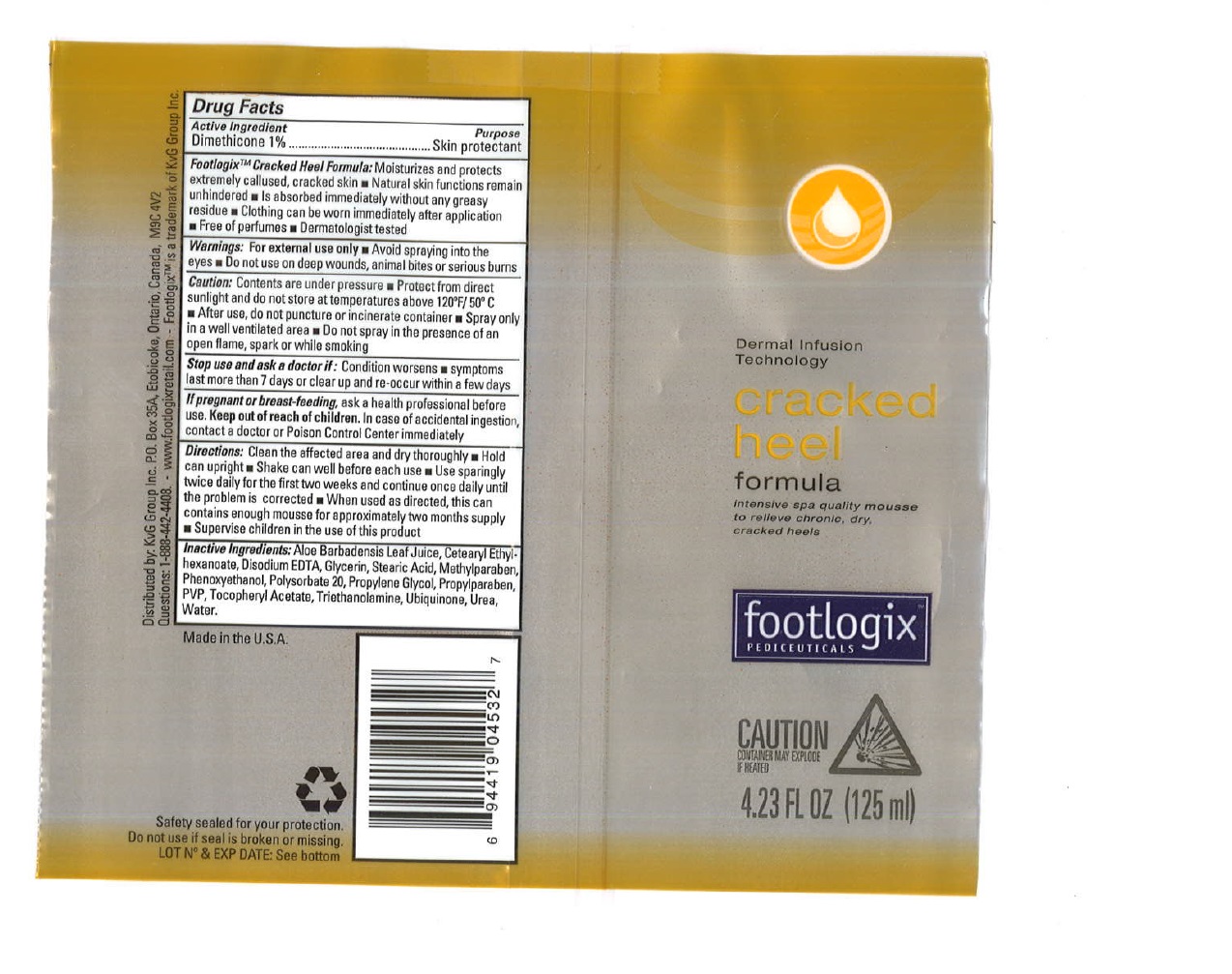
Footlogix TM
Cracked Heel Formula: Moisturizes and protects extremely callused, cracked skin
- Natural skin functions remain unhindered
- Is absorbed immediately without any greasy residue
- Clothing can be worn immediately after application
- Free of perfumes
- Dermatologist tested
Warnings
For external use only
- Avoid spraying into the eyes
Caution:
Contents are under pressure
- Protect from direct sunlight and do not store at temperatures above 120ºF / 50ºC
- After use, do not puncture or incinerate container
- Spray only in a well ventilated area
- Do not spray in the presence of an open flame, spark or while smoking
Directions:
Clean the affected area and dry thoroughly
- Hold can upright
- Shake can well before each use
- Use sparingly twice daily for the first two weeks and continue once daily until the problem is corrected
- When used as directed, this can contains enough mousse for approximately two months supply
- Supervise children in the use of this product
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Butane, Cetearyl Ethylhexanoate, Disodium EDTA, Glycerin, Stearic Acid, Methylparaben, Oenothera Biennis Oil, Phenoxyethanol, Polysorbate 20, Propane, Propylene Glycol, Propylparaben, PVP, Tocopheryl Acetate, Triethanolamine, Ubiquinone, Urea, Water.