Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 6-12 hours
Warnings
Do not use
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Directions
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
|---|---|---|
| adults and children 12 years of age or older | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | ask a doctor | ask a doctor |
Inactive Ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, dicalcium phosphate, D&C Yellow #10 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, hypromellose, lactose anhydrous, magnesium stearate, microcrystalline cellulose, PEG 8000, sodium benzoate, stearic acid, tartaric acid, titanium dioxide
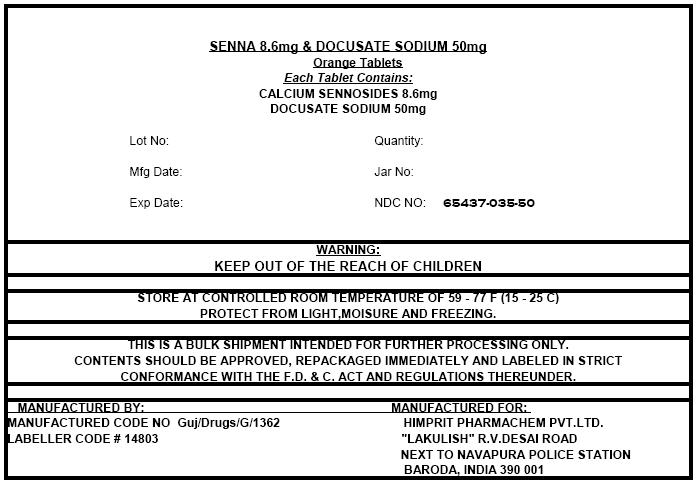
PRINCIPAL DISPLAY PANEL - Shipping Label
SENNA 8.6mg & DOCUSATE SODIUM 50mg
Orange Tablets
Each Tablet Contains:
CALCIUM SENNOSIDES 8.6mg
DOCUSATE SODIUM 50mg
| Lot No: | Quantity: |
| Mfg Date: | Jar No: |
| Exp Date: | NDC NO: 65437-035-50 |
WARNING:
KEEP OUT OF THE REACH OF CHILDREN
STORE AT CONTROLLED ROOM TEMPERATURE OF 59 - 77 F (15 - 25 C)
PROTECT FROM LIGHT,MOISURE AND FREEZING.
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE F.D. & C. ACT AND REGULATIONS THEREUNDER.
MANUFACTURED BY:
MANUFACTURED CODE NO Guj/Drugs/G/1362
LABELLER CODE # 14803
MANUFACTURED FOR:
HIMPRIT PHARMACHEM PVT.LTD.
"LAKULISH" R.V.DESAI ROAD
NEXT TO NAVAPURA POLICE STATION
BARODA, INDIA 390 001