DESCRIPTION:
Safe-Guard® (fenbendazole) Paste 10% contains the active anthelmintic, fenbendazole. The chemical name of fenbendazole is methyl 5-(phenyl-thio)-2-benzimidazole carbamate.
The CAS Registry Number is 43210-67-9.
The chemical structure is:
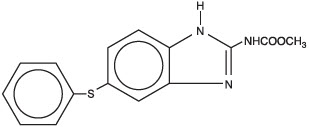
Each gram of Safe-Guard® Paste 10% contains 100 mg of fenbendazole and is flavored with artificial apple-cinnamon liquid.
ACTIONS:
The antiparasitic action of Safe-Guard® Paste 10% is believed to be due to the inhibition of energy metabolism in the parasite.
INDICATIONS:
Horses: For the treatment and control of large strongyles (Strongylus edentatus, S. equinus, S. vulgaris), encysted early 3rd stage (hypobiotic), late 3rd stage and 4th stage cyathostome larvae, small strongyles, pinworms (Oxyuris equi), ascarids (Parascaris equorum), and for the control of arteritis caused by 4th stage larvae of Strongylus vulgaris in horses.
Beef and Dairy Cattle: For the treatment and control of: Lungworms: Adult Dictyocaulus viviparus; Stomach worms: Adult brown stomach worms (Ostertagia ostertagi), Adult and fourth stage larvae barberpole worms (Haemonchus contortus), fourth stage larvae barberpole worms (H. placei), and Adult and fourth stage larvae small stomach worms (Trichostrongylus axei); Intestinal worms (Adult and fourth stage larvae): hookworms (Bunostomum phlebotomum), thread-necked intestinal worms (Nematodirus helvetianus), small intestinal worms (Cooperia punctata & C. oncophora), bankrupt worms (Trichostrongylus colubriformis), and nodular worms (Oesophagostomum radiatum).
One syringe deworms 4 (1,000 lb) cattle or 4 (1,000 lb) horses at a dose of 5 mg/kg or 4 (500 lb) horses at a dose of 10 mg/kg to treat ascarid infections.
PRECAUTIONS:
Horses: Side effects associated with Safe-Guard® Paste 10% could not be established in well-controlled safety studies in horses with single doses as high as 454 mg/lb (1,000 mg/kg) and 15 consecutive daily doses of 22.7 mg/lb (50 mg/kg). At higher dose levels, the lethal action of fenbendazole may cause the release of antigens by the dying parasites. This phenomenon may result in either a local or systemic hypersensitivity reaction varying in severity from itching or a rash to increased respiration and collapse. A veterinarian should be consulted if this type of reaction is suspected.
Safe-Guard® Paste 10% has been evaluated for safety in pregnant mares during all stages of gestation with doses as high as 11.4 mg/lb (25 mg/kg) and in stallions with doses as high as 11.4 mg/lb (25 mg/kg). No adverse effects on reproduction were detected. The recommended dose for control of 4th stage Strongylus vulgaris larvae, 4.6 mg/lb (10 mg/kg) daily for 5 consecutive days, has not been evaluated for safety in stallions or pregnant mares.
DOSAGE:
Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
Horses: Safe-Guard® Paste 10% is administered orally at a rate of 2.3 mg/lb (5 mg/kg) for the control of large strongyles, small strongyles, and pinworms. Each mark on the plunger rod corresponds to a dose of 2.3 mg/lb (5 mg/kg) for 250 lb body weight.
For foals and weanlings (less than 18 months of age) where ascarids are a common problem, the recommended dose of 4.6 mg/lb (10 mg/kg), or two marks, will deworm a 250 Ib horse.
For control of hypobiotic (encysted early 3rd stage), late 3rd stage, and 4th stage cyathostome larvae, as well as 4th stage Strongylus vulgaris larvae, the recommended dose is 4.6 mg/lb (10 mg/kg) daily for 5 consecutive days; administer two marks for each 250 Ib body weight per day.
Withdrawal Periods and Residue Warnings: Milk taken during treatment and for 96 hours after the last treatment must not be used for human consumption. Cattle must not be slaughtered for human consumption within 8 days following last treatment with this drug product. Not for use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
WARNINGS: NOT FOR USE IN HUMANS. KEEP OUT OF REACH OF CHILDREN. The Safety Data Sheet (SDS) contains more detailed occupational safety information. For customer service, adverse effects reporting, and/or a copy of the SDS, call 1-800-211-3573. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDAVETS, or http://www.fda.gov/reportanimalae.
OTHER WARNINGS: Do not use in horses intended for human consumption.
Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers. Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance. Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method). A decrease in a drug's effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
DIRECTIONS FOR USE:
- Determine the weight of the animal.
- Remove syringe tip.
- Turn the dial ring until the edge of the ring nearest the tip lines up with zero.
- Fully depress plunger and discard expelled paste. Syringe is ready for dosing.
- Each mark on the plunger rod corresponds to a dose of 2.3 mg/lb (5 mg/kg) for 250 lb body weight, or a dose of 4.6 mg/lb (10 mg/kg) for 125 lb body weight (the 10 mg/kg dose is for horses only).
Dial the ring edge nearest the tip back by the calculated number of marks on the plunger rod corresponding to the animal's body weight.
Examples:
Body Weight Number of marks for 2.3 mg/lb (5 mg/kg) dose for cattle or horses Number of marks for 4.6 mg/lb (10 mg/kg) dose for horses only 250 lb 1 2 500 lb 2 4 750 lb 3 6 1000 lb 4 8 1500 lb 6 12 - The animal's mouth should be free of food. Insert nozzle of syringe through the interdental space and deposit the paste on the back of the tongue by depressing the plunger.
- Repeat steps 1, 5 and 6 for each additional animal.
Fenbendazole (active ingred.) made in China. Formulated in France.
Distributed by:
Intervet Inc. (d/b/a Merck Animal Health)
Madison, NJ 07940
Approved by FDA under NADA # 132-872
Approved by FDA under NADA # 120-648
©2020 Intervet Inc., a subsidiary of Merck & Co. Inc.
Rev. 09/20
MERCK
Animal Health
PRINCIPAL DISPLAY PANEL - 25 g Syringe Carton
MERCK
Animal Health
safe-guard®
(fenbendazole)
Equine
Dewormer
25 gram Paste 10%
(100 mg/g)
MERCK
Animal Health
PRINCIPAL DISPLAY PANEL - 92 g Syringe Carton
safe-guard®
(fenbendazole)
Dewormer
for Beef & Dairy Cattle and Horses
92 g Paste 10% (100 mg/g)
One syringe deworms 4 (1,000 lb) cattle or 4 (1,000 lb)
horses at a dose of 5 mg/kg or 4 (500 lb) horses
at a dose of 10 mg/kg to treat ascarid infections.
Net Weight 92 g (3.2 oz)
MERCK
Animal Health

