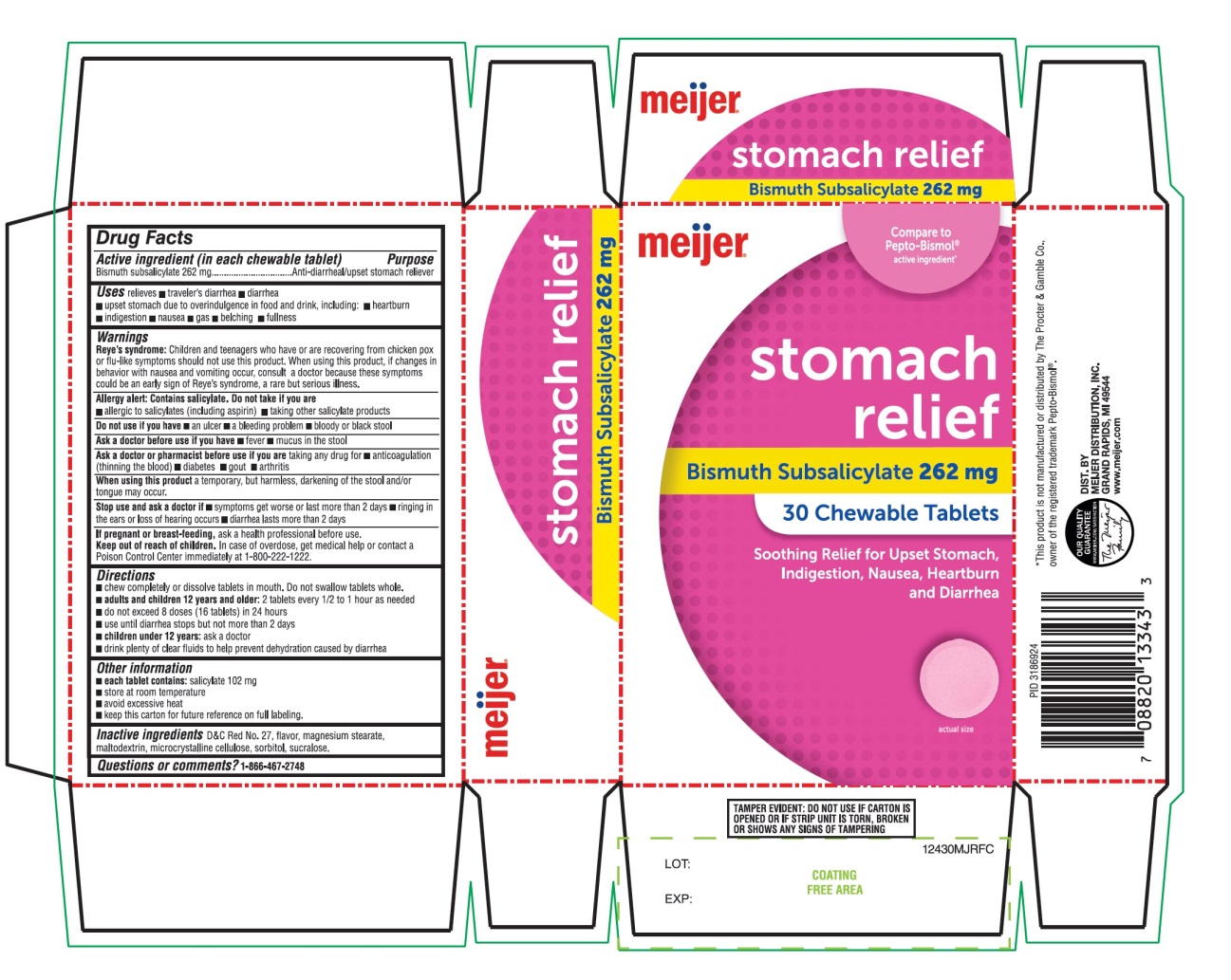
Uses
relieves:
- ▪
- travelers’ diarrhea
- ▪
- diarrhea
- ▪
- upset stomach due to overindulgence in food and drink, including:
- ▪
- heartburn
- ▪
- indigestion
- ▪
- nausea
- ▪
- gas
- ▪
- belching
- ▪
- fullness
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- ▪
- allergic to salicylates (including aspirin)
- ▪
- taking other salicylate products
Do not use if you have
- ▪
- an ulcer
- ▪
- a bleeding problem
- ▪
- bloody or black stool
Ask a doctor before use if you have
- ▪
- fever
- ▪
- mucus in the stool
Ask a doctor or pharmacist before use if you are taking any drug for
- ▪
- anticoagulation (thinning of the blood)
- ▪
- diabetes
- ▪
- gout
- ▪
- arthritis
Directions
- ▪
- chew completely or dissolve tablets in mouth. Do not swallow tablets whole.
- ▪
- adults and children 12 years and older: 2 caplets every 1/2 to 1 hour as needed
- ▪
- do not exceed 8 doses (16 caplets) in 24 hours
- ▪
- use until diarrhea stops but not more than 2 days
- ▪
- children under 12 years: ask a doctor
- ▪
- drink plenty clear fluids to help prevent dehydration caused by diarrhea.
Other information
- ▪
- each caplet contains: salicylate 102 mg
- ▪
- store at room temperature.
- ▪
- avoid excessive heat
- ▪
- keep this carton for future reference on full labeling
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF STRIP UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TEMPERING.
Inactive ingredients
D&C Red No. 27, flavor, magnesium stearate, maltodextrin, microcrystalline cellulose, sorbitol, sucralose.
Principal Display
meijer®
BDC# 41250-878-30
Compare To Pepto-Bismol® Caplets active ingredient*
Stomach relief
Bismuth Subsalicylate 262 mg
Soothing relief for Upset Stomach, Indigestion, Nausea, Heartburn and Diarrhea.
30 Chewable Tablets
OUR QUALITY GUARANTEE
DIST. BY
MEIJER DISTRIBUTION, INC
GRAND RAPIDS, MI 49544
*This product is not manufactured or distributed by The Procter & Gamble Co., owner of the registered trademark Pepto-Bismol®.