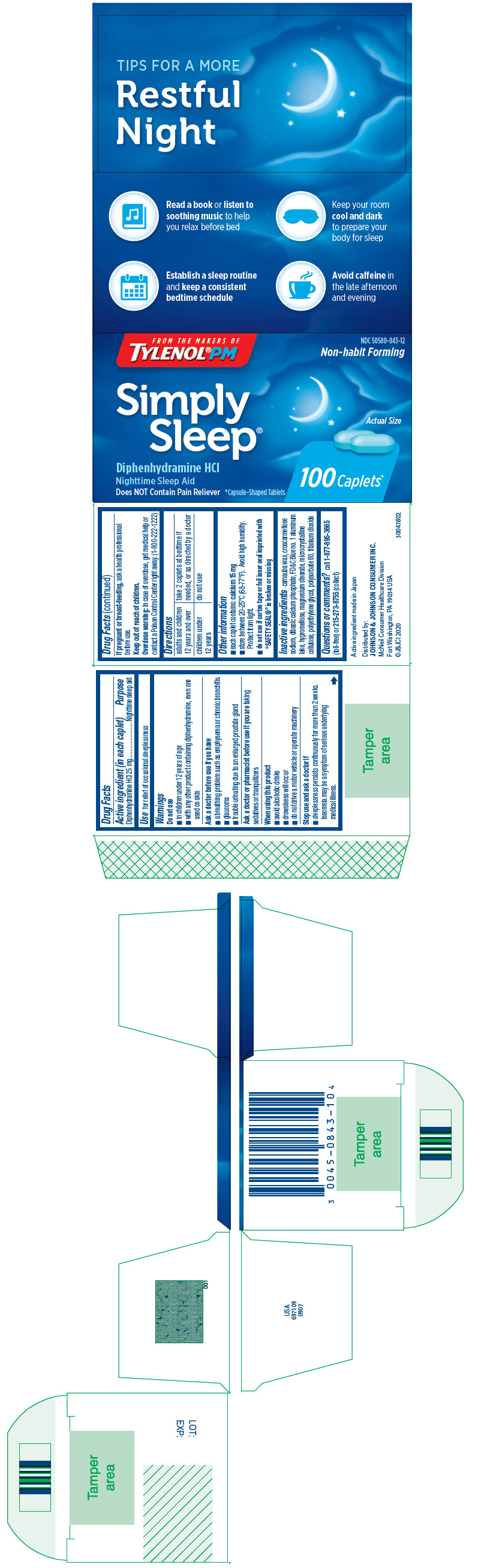
Active ingredient (in each caplet)
Diphenhydramine HCl 25 mg
Purpose
Nighttime sleep aid
Use
for relief of occasional sleeplessness
Warnings
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
- avoid alcoholic drinks
- drowsiness will occur
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Overdose warning
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
| adults and children 12 years and over | take 2 caplets at bedtime if needed, or as directed by a doctor |
| children under 12 years | do not use |
Other information
- each caplet contains:
calcium 15 mg
- store between 20-25°C (68-77°F). Avoid high humidity. Protect from light.
-
do not use if carton tape or foil inner seal imprinted with "SAFETY SEAL®" is broken or missing
Inactive ingredients
carnauba wax, croscarmellose sodium, dibasic calcium phosphate, FD&C Blue no. 1 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide
Questions or comments?
call
1-877-895-3665 (toll-free) or
215-273-8755 (collect)
PRINCIPAL DISPLAY PANEL
FROM THE MAKERS OF
TYLENOL
®PM
NDC 50580-843-12
Non-habit Forming
Simply
Sleep
®
Diphenhydramine HCl
Nighttime Sleep Aid
Does NOT Contain Pain Reliever
*Capsule-Shaped Tablets
Actual Size
100 Caplets*
Johnson & Johnson Consumer Inc.