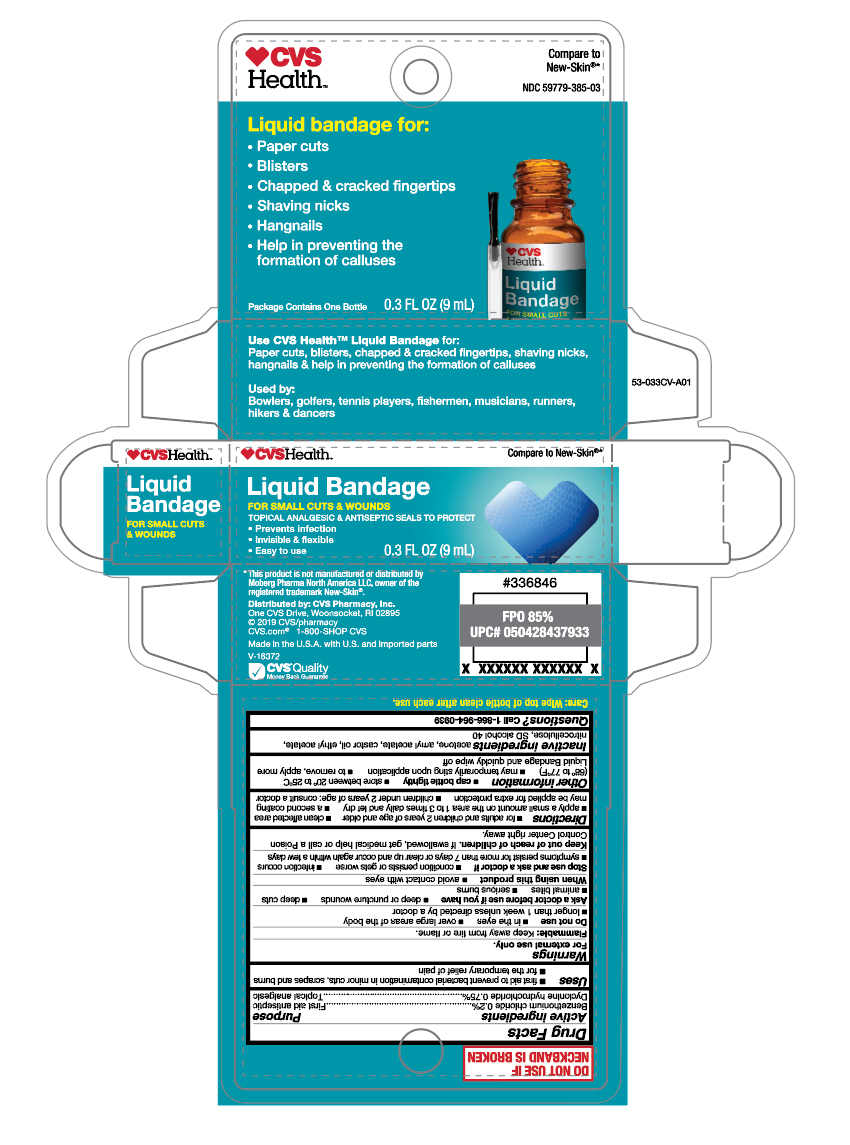
Active ingredients
Benzethonium chloride 0.2%
Dyclonine hydrochloride 0.75%
Purpose
First aid antiseptic
Topical analgesic
Uses
- first aid to prevent bacterial contamination in minor cuts, scrapes and burns
- for the temporary relief of pain
Warnings
For external use only.
Flammable:
Keep away from fire or flame.
Do not use
- in the eyes
- over large areas of the body
- longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- deep or puncture wounds
- deep cuts
- animal bites
- serious burns
Stop use and ask a doctor if
- conditions persists or gets worse
- infection occurs
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or call a Poison Control Center right away.
Directions
- for adults and children 2 years of age and older
- clean affected area
- apply a small amount on the area 1 to 3 times daily and let dry
- a second coating may be applied for extra protection
- children under 2 years of age: consult a doctor
Other information
-
cap bottle tightly
- store between 20° to 25°C (68° to 77°F)
- may temporarily sting upon application
- to remove, apply more Liquid Bandage and quickly wipe off
Inactive ingredients
acetone, amyl acetate, castor oil, ethyl acetate, nitrocellulose, SD slcohol 40
Questions
Call 1-866-964-0939
Principal Display Panel
CVS Health
Liquid Bandage for:
- Paper cuts
- Blisters
- Chapped & cracked fingertips
- Shaving nicks
- Hangnails
- Help in preventing the formation of calluses
Package Contains One Bottle
0.3 FL OZ (9mL)