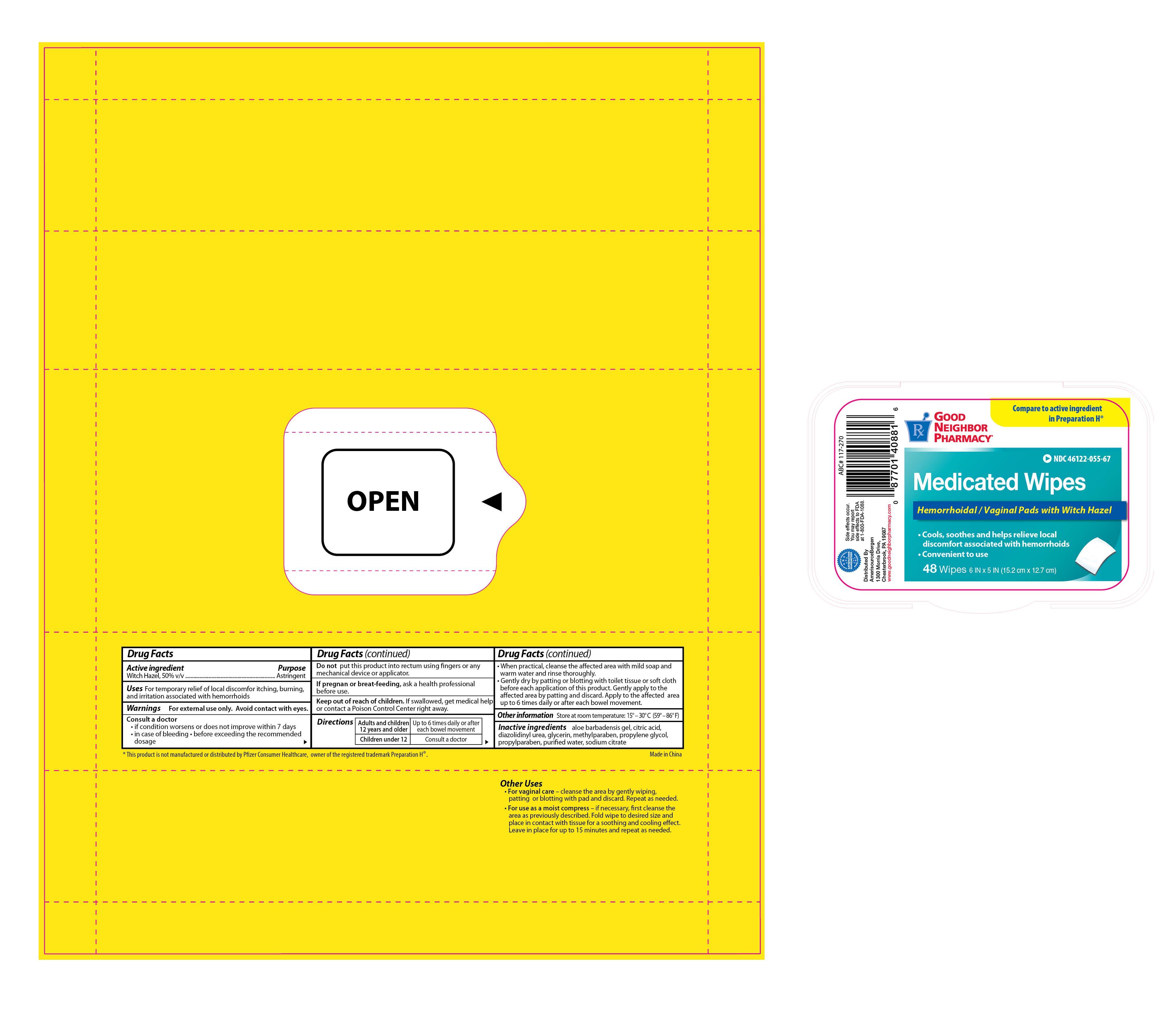
Active ingredient Purpose
Witch Hazel, 50% v/v...................................................Astringent
Uses For temporary relief of local discomfort itching, burning, and irritation associated with hemorrhoids
Consult a doctor
- if condition worsens or does not improve within 7 days
- in case of bleeding
- before exceeding the recommended dosage
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 12 years and older: up to 6 times daily or after each bowel movement
- Children under 12: consult a doctor
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- Gently dry by patting or blotting with toilet tissue or soft cloth before each application of this product. Gently apply to the affected area by patting and discard. Apply to the affected area up to 6 times daily or after each bowel movement.