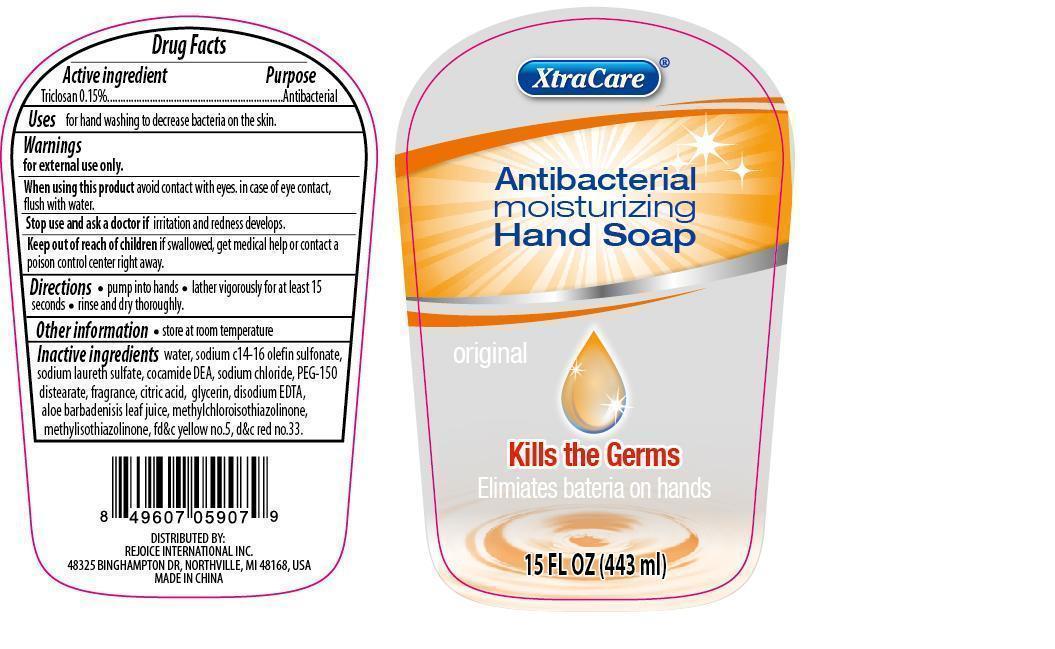
XTRACARE ANTIBACTERIAL HAND CLEANSE ORIGINAL- triclosan soap
Rejoice International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient Purpose
Triclosan 0.15%................................Antibacterial
Antibacterial moisturizing Hand Soap
Original
Kills the Germs
Elimiates bacteria on hands
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Uses
for hand washing to decrease bacteria on the skin.
Warnings
for external use only
When using this product avoid contact with eyes. In case of eye contact, flush with water.
Stop use and ask a doctor if irritation and redness develops.
Directions
- pump into hands
- lather vigorously for at least 15 seconds
- rinse and dry thoroughly.
Inactive Ingredients
water, sodium c14-16 olefin sulfonate, sodium laureth sulfate, cocamide DEA, sodium chloride, PEG-150 distearate, fragrance, citric acid, glycerin, disodium EDTA, aloe barbadensis leaf juice, methylchloroisothiazolinone, methylisothiazolinone, fd&c yellow no. 5, d&c red no. 33.
Other information
- store at room temperature
DISTRIBUTED BY:
REJOICE INTERNATIONAL, INC.
48325 BINGHAMPTON DR., NORTHVILLE, MI 48168, USA
MADE IN CHINA
Rejoice International, Inc.