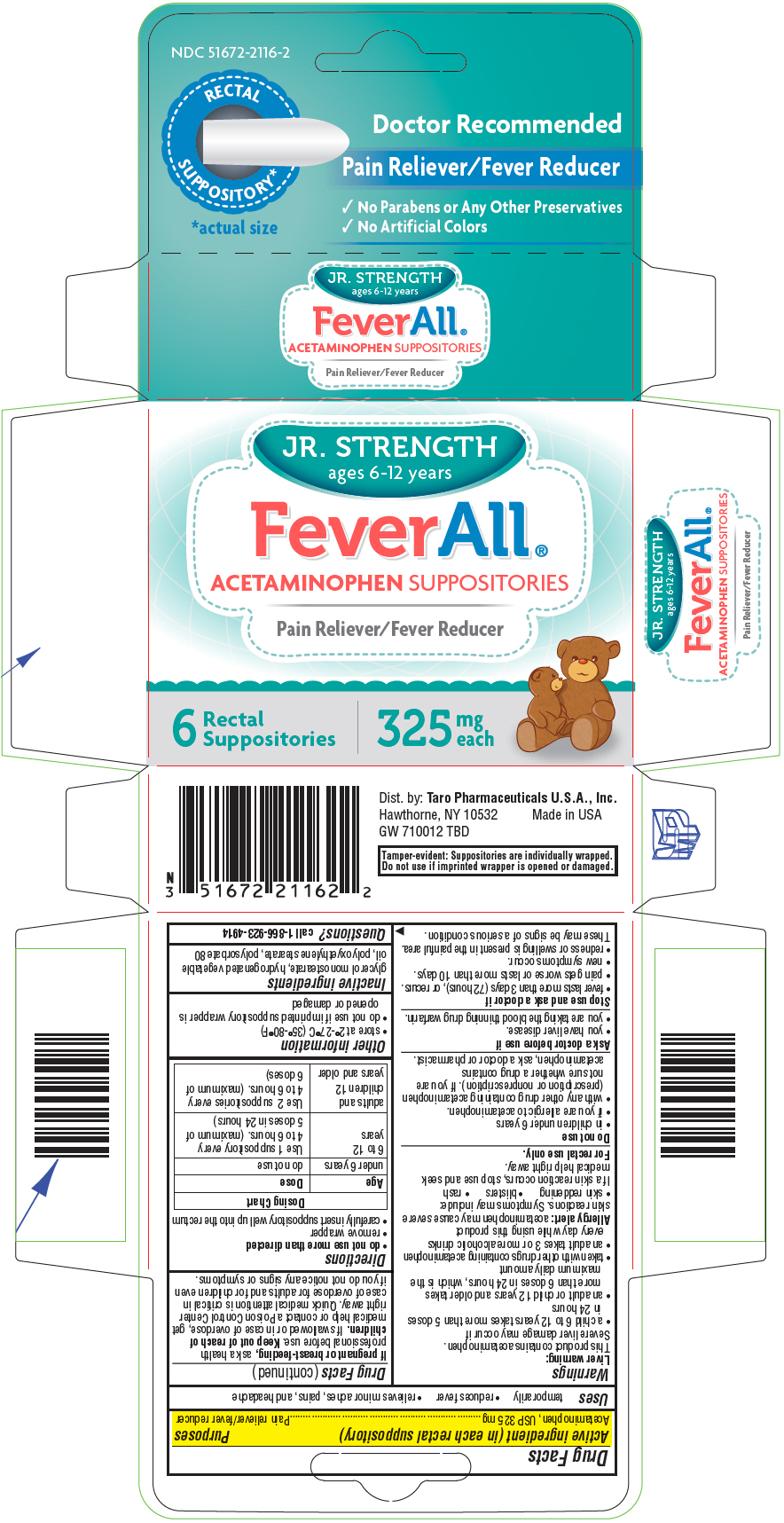
Warnings
Liver warning
This product contains acetaminophen.
Severe liver damage may occur if
- a child 6 to 12 years takes more than 5 doses in 24 hours
- an adult or child 12 years and older takes more than 6 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- an adult takes 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
For rectal use only.
Do not use
- in children under 6 years
- if you are allergic to acetaminophen.
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- fever lasts more than 3 days (72 hours), or recurs.
- pain gets worse or lasts more than 10 days.
- new symptoms occur.
- redness or swelling is present in the painful area.
These may be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. If swallowed or in case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical in case of overdose for adults and for children even if you do not notice any signs or symptoms.
Directions
- do not use more than directed
- remove wrapper
- carefully insert suppository well up into the rectum
| Age | Dose |
|---|---|
| under 6 years | do not use |
| 6 to 12 years | Use 1 suppository every 4 to 6 hours. (maximum of 5 doses in 24 hours) |
| adults and children 12 years and older | Use 2 suppositories every 4 to 6 hours. (maximum of 6 doses) |
Other information
- store at 2°-27°C (35°-80°F)
- do not use if imprinted suppository wrapper is opened or damaged
Inactive ingredients
glycerol monostearate, hydrogenated vegetable oil, polyoxyethylene stearate, polysorbate 80
PRINCIPAL DISPLAY PANEL - 325 mg Suppository Blister Pack Carton
NDC 51672-2116-2
RECTAL
SUPPOSITORY*
*actual size
Doctor Recommended
Pain Reliever/Fever Reducer
- ✓
- No Parabens or Any Other Preservatives
- ✓
- No Artificial Colors
JR. STRENGTH
ages 6-12 years
FeverAll®
ACETAMINOPHEN SUPPOSITORIES
Pain Reliever/Fever Reducer
6
Rectal
Suppositories
325
mg
each