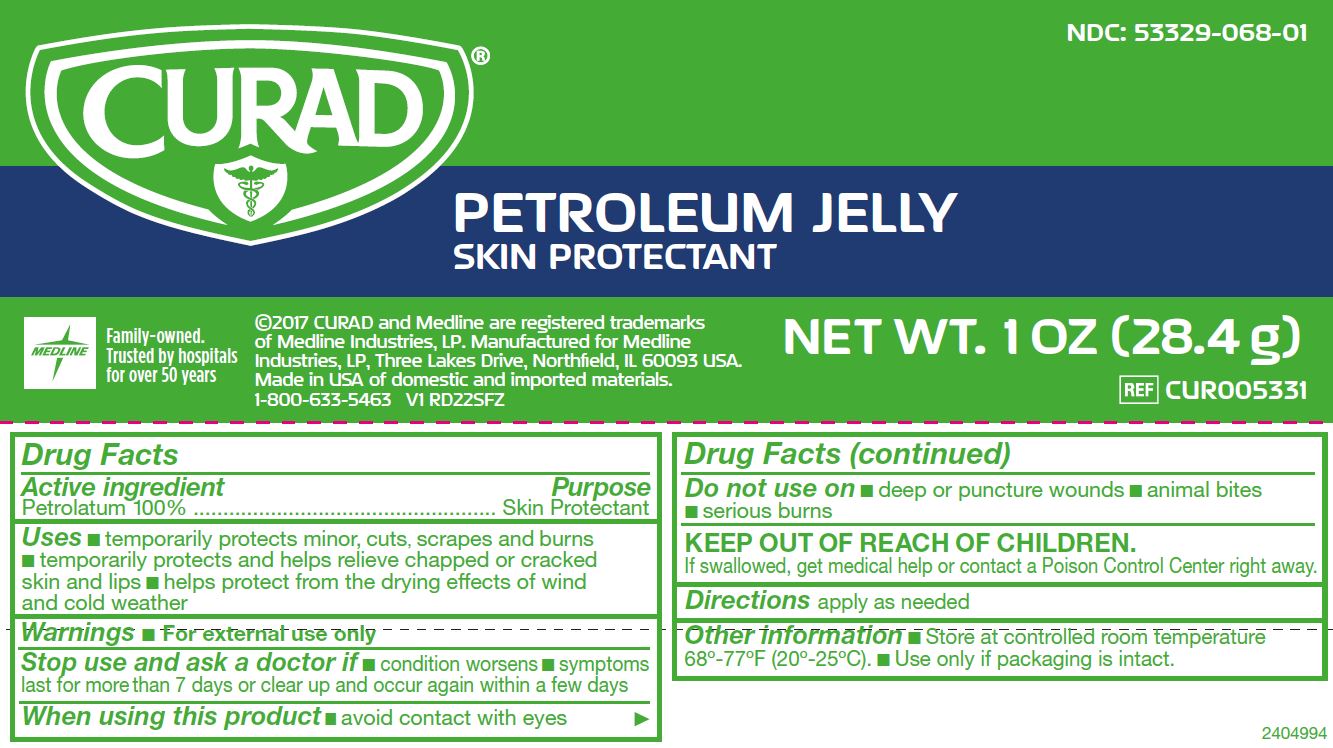
PETROLEUM- white petrolatum jelly
Medline Industries, LP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each gram)
Petrolatum 100%
Uses
- temporarily protects minor: cuts, scrapes and burns
- temporarily protects and helps relieve chapped or cracked skin and lips
- helps protect from the drying effects of wind and cold weather
Warnings
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens
- symptoms last for more than 7 days or clear up and occur again within a few days
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
apply as needed.
Other information
- Store at controlled room temperature 68º-77ºF (20º-25ºC).
- Use only if packaging is intact.
Manufacturing Information
Manufactured for:
Medline Industries, LP
Three Lakes Drive, Northfield, IL 60093 USA
Made in USA of Domestic and Imported Materials
www.curad.com
1-800-633-5463
REF: CUR005331
V1 RD22SFZ
Package Label