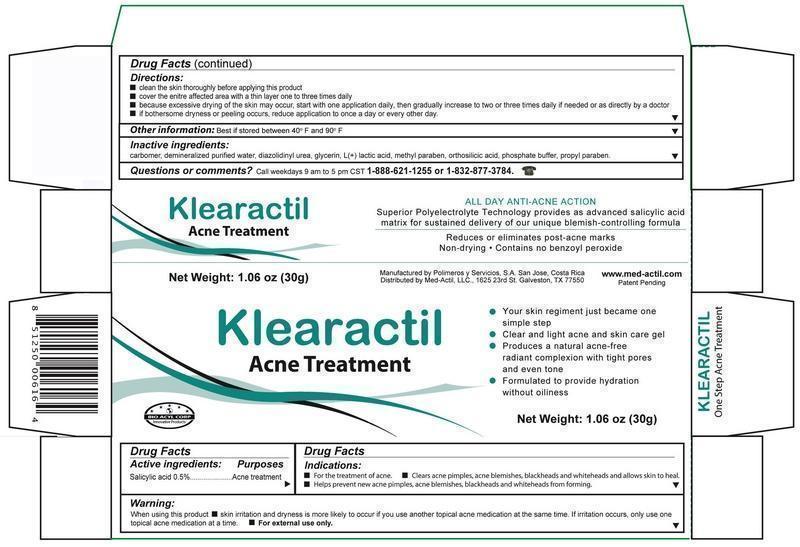
Detail showing Active Ingredient from package
Active ibgredients_001.jpg

Information shown both on container label and paclage
Detail showing Inactive ingredient from package
Inactive ingredients.jpg

Information shown both on container label and paclage
Detail showing Purpose within Active Ingredients from package
Purpose.jpg

Information shown both on container label and package
Detail showing Instructions for use and named Directions from package
indications_001.jpg

Information shown both on container label and package
Detail showing Dosage and Administration within Directions from package
Dosage and administration.jpg

Information shown both on container lable and package
Detail showing Warnings from package
Warnings.jpg

nformation shown both on container label and package
Detail showing Keep Out of Reach of Children within Warnings from package
Keep out of reach of Childreb.jpg

Information shown on package
Detail showing Questions from package
Questions.jpg

Information shown both on container label and package