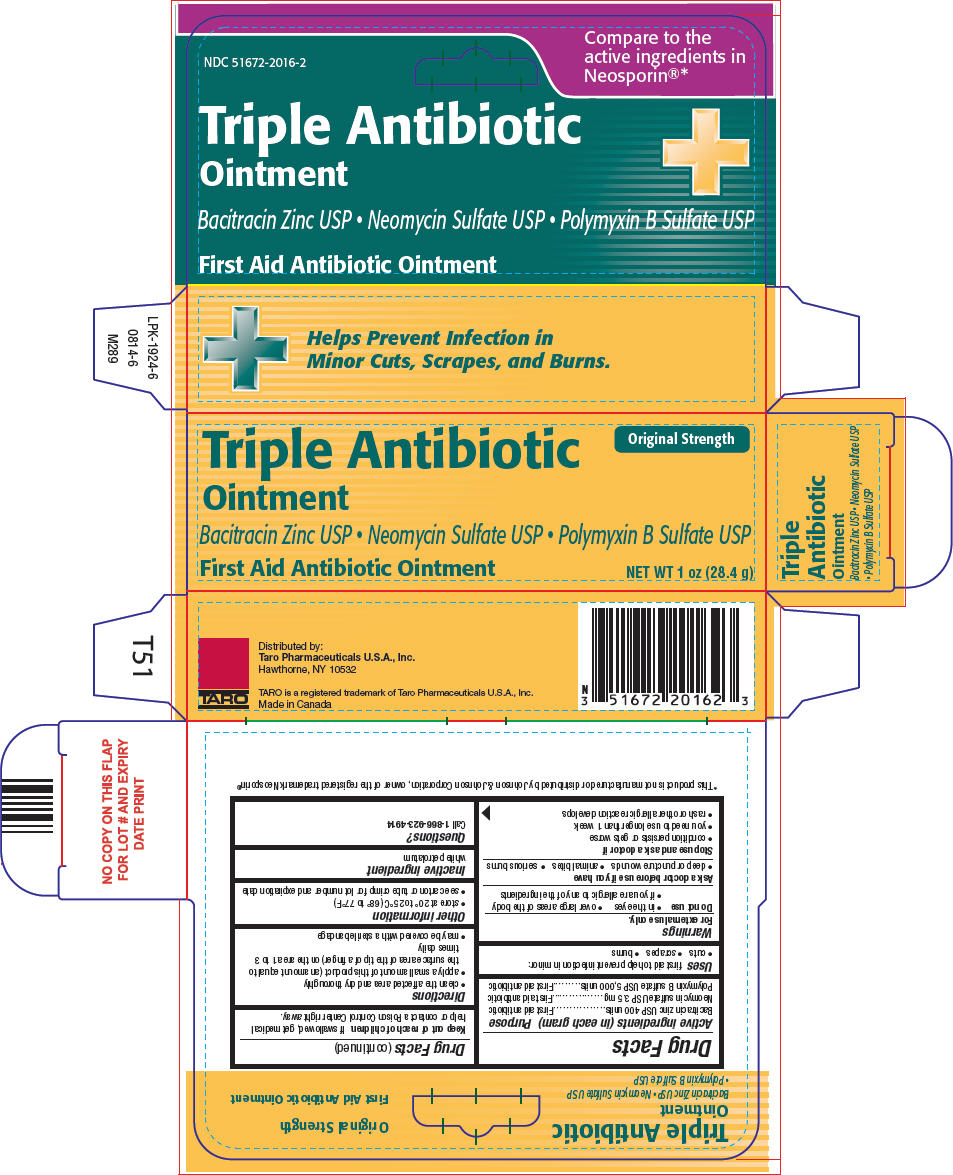
TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointment
Taro Pharmaceuticals U.S.A., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients (in each gram) | Purpose |
| Bacitracin zinc USP 400 units | First aid antibiotic |
| Neomycin sulfate USP 3.5 mg | First aid antibiotic |
| Polymyxin B sulfate USP 5,000 units | First aid antibiotic |
Uses
first aid to help prevent infection in minor:
Warnings
For external use only.
Do not use
- in the eyes
- over large areas of the body
- if you are allergic to any of the ingredients
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition persists or gets worse
- you need to use longer than 1 week
- rash or other allergic reaction develops
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the affected area and dry thoroughly
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Other information
- store at 20° to 25°C (68° to 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredient
white petrolatum
Questions?
Call 1-866-923-4914
Distributed by:
Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
Triple Antibiotic
Ointment
Original Strength
Bacitracin Zinc USP • Neomycin Sulfate USP • Polymyxin B Sulfate USP
First Aid Antibiotic Ointment
NET WT 1 oz (28.4 g)
Taro Pharmaceuticals U.S.A., Inc.