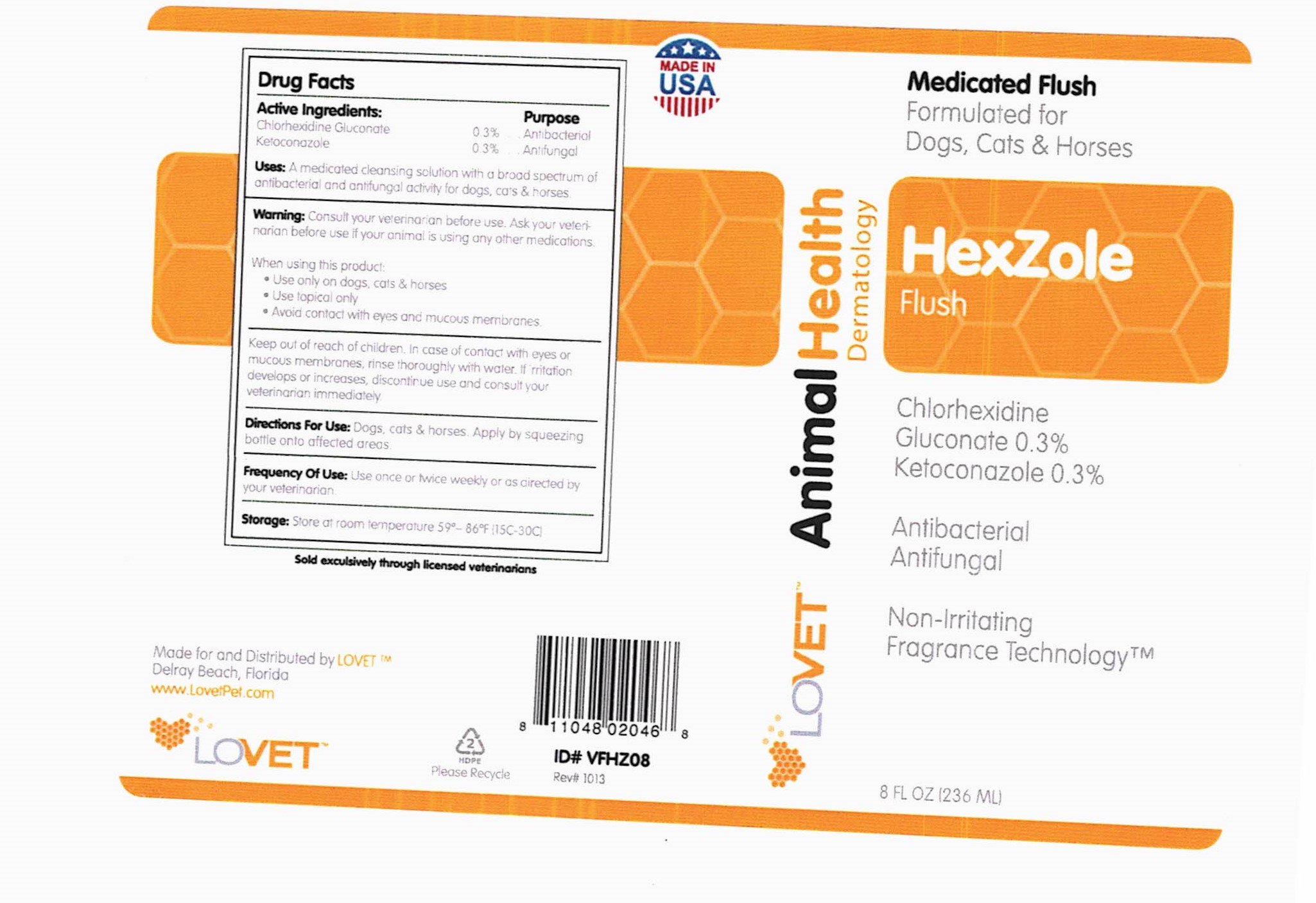
Active Ingredients: Purpose
Chlorhexidine Gluconate …. 0.3%. . . . ...Antibacterial
Ketoconazole ………………...... 0.3% . .......Antifungal
Uses: A medicated cleansing solution with a broad spectrum of antibacterial and antifungal activity for dogs, cats & horses.
Warning: Consult your veterinarian before use. Ask your veterinarian before use if your animal is using any other medications.
When using this product:
- Use only on dogs, cats & horses
- Use topical only
- Avoid contact with eyes and mucous membranes.
Keep out of reach of children. In case of contact with eyes or mucous membranes, rinse thoroughly with water. If irritation develops or increases, discontinue use and consult your veterinarian immediately.
Directions For Use: Dogs, cats and horses. Apply by squeezing bottle onto affected areas.
Frequency Of Use: Use once or twice weekly or as directed by your veterinarian.