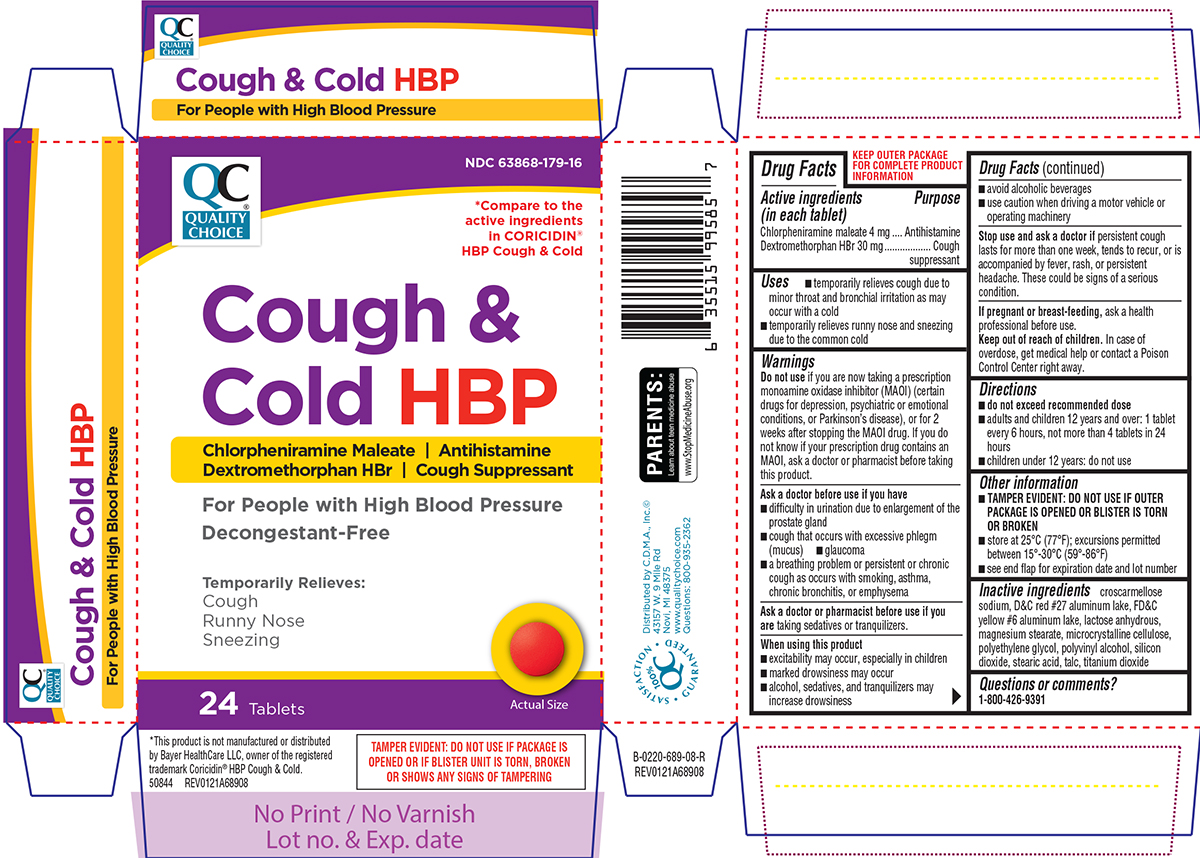
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- temporarily relieves runny nose and sneezing due to the common cold
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- cough that occurs with excessive phlegm (mucus)
- glaucoma
- a breathing problem or persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
Directions
- do not exceed recommended dose
- adults and children 12 years and over: 1 tablet every 6 hours, not more than 4 tablets in 24 hours
- children under 12 years: do not use
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, D&C red #27 aluminum lake, FD&C yellow #6 aluminum lake, lactose anhydrous, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, stearic acid, talc, titanium dioxide
Principal display panel
QC®
QUALITY
CHOICE
NDC 63868-179-16
*Compare to the active ingredients in CORICIDIN® HBP Cough & Cold
Cough &
Cold HBP
Chlorpheniramine Maleate | Antihistamine
Dextromethorphan HBr | Cough Suppressant
For People with High Blood Pressure
Decongestant-Free
Temporarily Relieves:
Cough
Runny Nose
Sneezing
24 Tablets
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Coricidin® HBP Cough & Cold.
50844 REV0121A68908
Distributed by C.D.M.A., Inc.©
43157 W. 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions: 800-935-2362
PARENTS:
Learn about teen medicine abuse
www.StopMedicineAbuse.org
Quality Choice 44-689