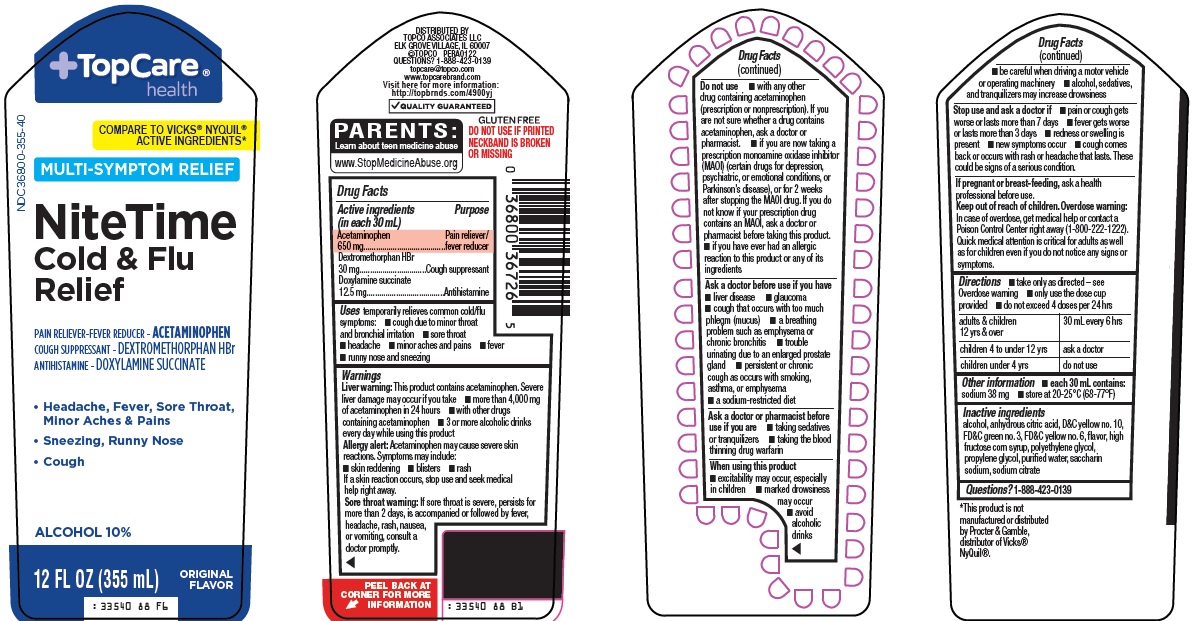
Active ingredients (in each 30 mL)
Acetaminophen 650 mg
Dextromethorphan HBr 30 mg
Doxylamine succinate 12.5 mg
Uses
temporarily relieves common cold/flu symptoms:
- •
- cough due to minor throat and bronchial irritation
- •
- minor aches and pains
- •
- headache
- •
- fever
- •
- runny nose and sneezing
- •
- sore throat
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 4,000 mg of acetaminophen in 24 hours
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, lasts for more than 2 days, occurs with or is followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- •
- liver disease
- •
- glaucoma
- •
- cough that occurs with too much phlegm (mucus)
- •
- persistent or chronic cough as occurs with smoking, asthma, or emphysema
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- trouble urinating due to an enlarged prostate gland
- •
- a sodium-restricted diet
Ask a doctor or pharmacist before use if you are
- •
- taking sedatives or tranquilizers
- •
- taking the blood thinning drug warfarin
When using this product
- •
- excitability may occur, especially in children
- •
- marked drowsiness may occur
- •
- avoid alcoholic drinks
- •
- be careful when driving a motor vehicle or operating machinery
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
Directions
- •
- take only as directed – see Overdose warning
- •
- only use the dose cup provided
- •
- do not exceed 4 doses per 24 hrs
|
adults & children 12 yrs & over |
30 mL every 6 hrs |
|
children 4 to under 12 yrs |
ask a doctor |
|
children under 4 yrs |
do not use |
Inactive ingredients
alcohol, anhydrous citric acid, D&C yellow no. 10, FD&C green no. 3, FD&C yellow no. 6, flavor, high fructose corn syrup, polyethylene glycol, propylene glycol, purified water, saccharin sodium, sodium citrate
Package/Label Principal Display Panel
TopCare® health
COMPARE TO VICKS® NYQUIL® ACTIVE INGREDIENTS
MULTI-SYMPTOM RELIEF
Nite Time Cold & Flu Relief
PAIN RELIEVER-FEVER REDUCER – ACETAMINOPHEN
COUGH SUPPRESSANT – DEXTROMETHORPHAN HBr
ANTIHISTAMINE – DOXYLAMINE SUCCINATE
• Headache, Fever, Sore Throat, Minor Aches & Pains
• Sneezing, Runny Nose
• Cough
ALCOHOL 10%
12 FL OZ (355 mL)
ORIGINAL FLAVOR