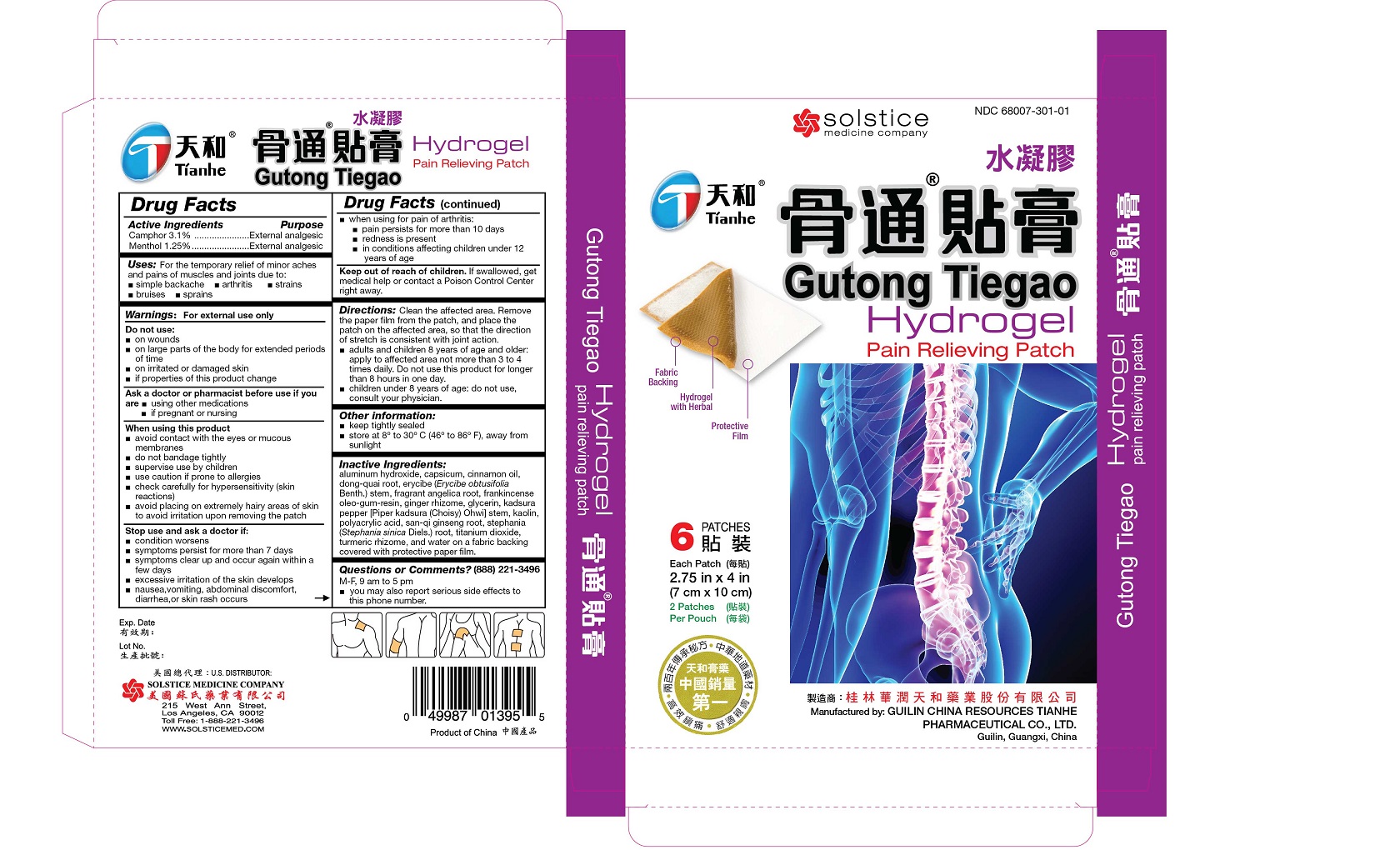
Uses
For the temporary relief of minor aches and pains of muscles and joints due to:
simple backache
arthritis
strains
bruises
sprains
Do not use
on wounds
on large parts of the body for extended periods of time
on irritated or damaged skin
if properties of this product change
When using this product
avoid contact with the eyes or mucous membranes
do not bandage tightly
supervise use by children
use caution if prone to allergies
check carefully for hypersensitivity (skin reactions)
avoid placing on extremely hairy areas of skin to avoid irritation upon removing the patch
Stop use and ask a doctor if
condition worsens
symptoms persist for more than 7 days
symptoms clear up and occur again within a few days
excessive irritation of the skin develops
nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
when using for pain of arthritis:
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years of age
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Clean the affected area. Remove the paper film from the patch, and place the patch on the affected area, so that the direction of stretch is consistent with joint action.
adults and children 8 years of age and older: apply to affected area not more than 3 to 4 times daily. Do not use this product for longer than 8 hours in one day.
children under 8 years of age: do not use, consult your physician
Inactive ingredients
aluminum hydroxide, capsicum, cinnamon oil, dong-quai root, erycibe (Erycibe obtusifolia Benth.) stem, fragrant angelica root, frankincense oleo-gum-resin, ginger rhizome, glycerin, kadsura pepper [Piper kadsura (Choisy) Ohwi] stem, kaolin, polyacrylic acid, san-qi ginseng root, stephania (Stephania sinica Diels.) root, titanium dioxide, turmeric rhizome, and water on a fabric backing covered with protective paper film.