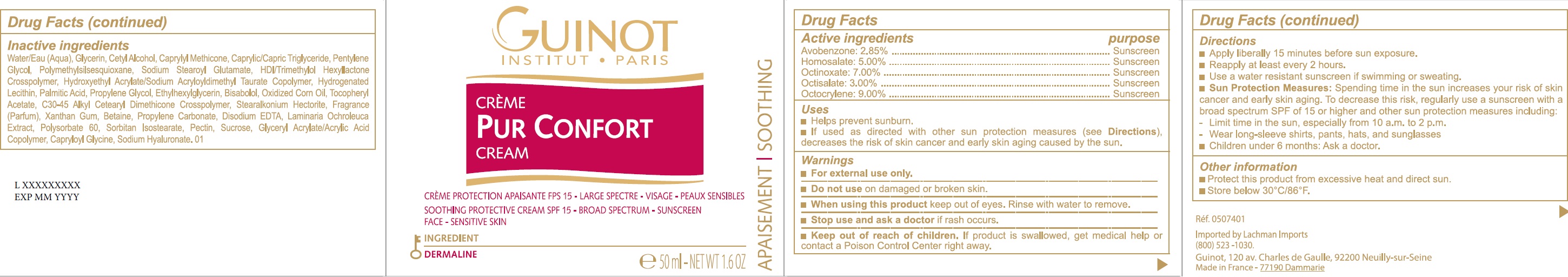
Active ingredients
AVOBENZONE: 2.85%
HOMOSALATE: 5.00%
OCTINOXATE: 7.00%
OCTISALATE: 3.00%
OCTOCRYLENE: 9.00%
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures:
- Limit time in the sun, especially from 10 a.m. to 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor.
Other information
- Protect this product from excessive heat and direct sun.
- Store below 30 C/86 F. oo
Inactive ingredients
Water/Eau (Aqua), Glycerin, Cetyl Alcohol, Caprylyl Methicone, Caprylic/Capric Triglyceride, Pentylene Glycol, Polymethylsilsesquioxane, Sodium Stearoyl Glutamate, HDI/Trimethylol Hexyllactone Crosspolymer, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Hydrogenated Lecithin, Palmitic Acid, Propylene Glycol, Ethylhexylglycerin, Bisabolol, Oxidized Corn Oil, Tocopheryl Acetate, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, Stearalkonium Hectorite, Fragrance (Parfum), Xanthan Gum, Betaine, Propylene Carbonate, Disodium EDTA, Laminaria Ochroleuca Extract, Polysorbate 60, Sorbitan Isostearate, Pectin, Sucrose, Glyceryl Acrylate/Acrylic Acid Copolymer, Capryloyl Glycine, Sodium Hyaluronate. 01