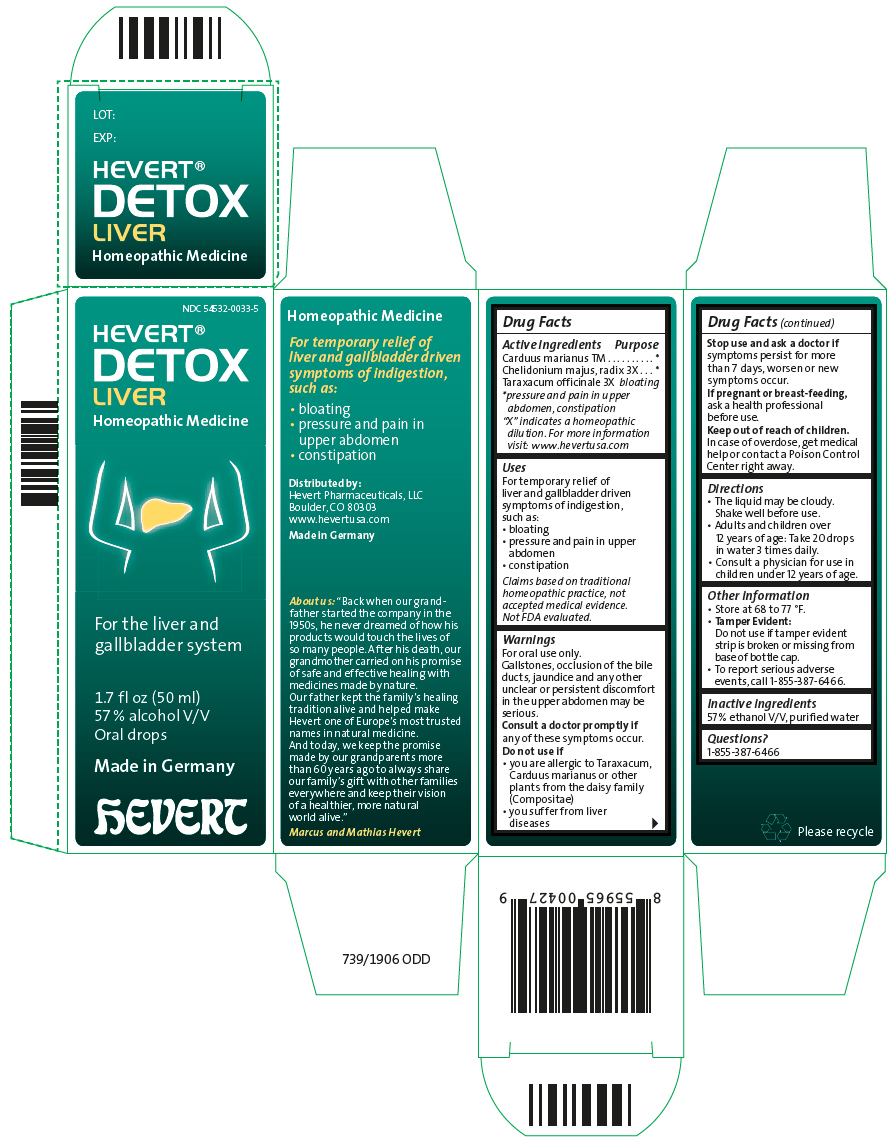
HEVERT DETOX LIVER- milk thistle, chelidonium majus root, and taraxacum officinale liquid
Hevert Arzneimittel GmbH & Co KG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
| Active ingredients | Purpose |
| "X" indicates a homeopathic dilution. For more information visit: www.hevertusa.com |
|
|
| Carduus marianus TM | * |
| Chelidonium majus, radix 3X | * |
| Taraxacum officinale 3X | bloating |
Uses
For temporary relief of liver and gallbladder driven symptoms of indigestion, such as:
- bloating
- pressure and pain in upper abdomen
- constipation
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings
For oral use only.
Gallstones, occlusion of the bile ducts, jaundice and any other unclear or persistent discomfort in the upper abdomen may be serious.
Consult a doctor promptly if any of these symptoms occur.
Do not use if
- you are allergic to Taraxacum, Carduus marianus or other plants from the daisy family (Compositae)
- you suffer from liver diseases
Stop use and ask a doctor if symptoms persist for more than 7 days, worsen or new symptoms occur.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away..
Directions
- The liquid may be cloudy. Shake well before use.
- Adults and children over 12 years of age: Take 20 drops in water 3 times daily.
- Consult a physician for use in children under 12 years of age.
Other information
- Store at 68 to 77 °F.
-
Tamper Evident:
Do not use if tamper evident strip is broken or missing from base of bottle cap.
- To report serious adverse events, call 1-855-387-6466.
Inactive ingredients
57 % ethanol V/V, purified water
Questions?
1-855-387-6466
Distributed by:
Hevert Pharmaceuticals, LLC
Boulder, CO 80303
PRINCIPAL DISPLAY PANEL - 50 ml Bottle Carton
NDC 54532-0033-5
HEVERT®
DETOX
LIVER
Homeopathic Medicine
For the liver and
gallbladder system
1.7 fl oz (50 ml)
57 % alcohol V/V
Oral drops
Made in Germany
hEVERT