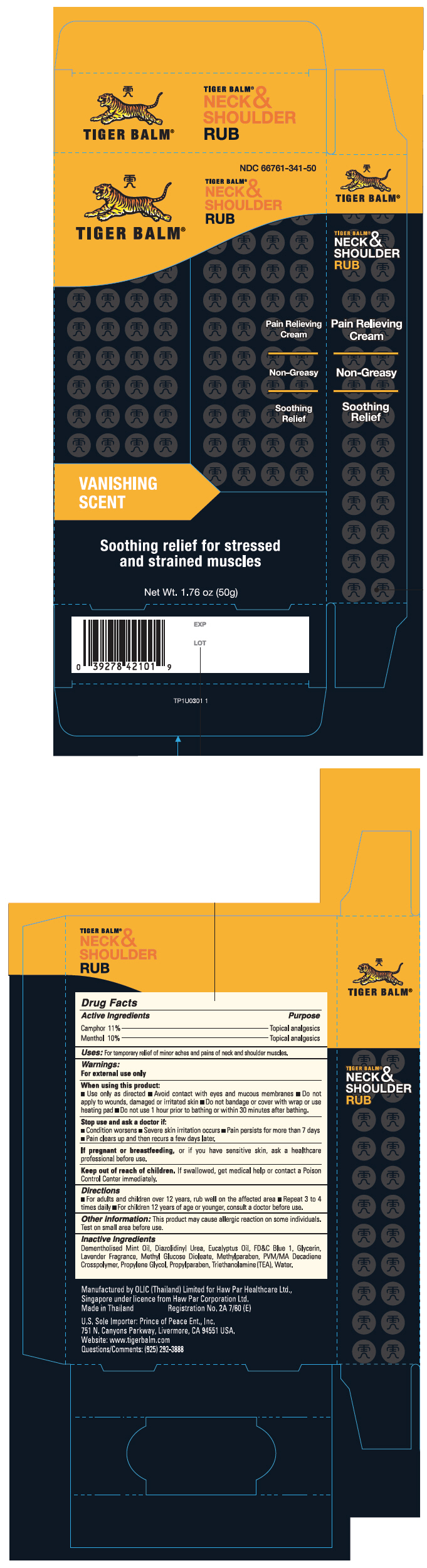
Active Ingredients
Camphor 11%, Menthol 10%
Purpose
Topical analgesics
Uses
For temporary relief of minor aches and pains of neck and shoulder muscles and joints
Warnings
For external use only
When using this product:
- Use only as directed
- Avoid contact with eyes and mucous membranes
- Do not apply to wounds, damaged or irritated skin
- Do not bandage or cover with wrap or use heating pad
- Do not use 1 hour prior to bathing or 30 minutes after bathing.
Stop use and ask a doctor if:
- Condition worsens
- Severe skin irritation occurs
- Pain persists for more than 7 days
- Pain clears up and then recurs a few days later.
If pregnant or breastfeeding, or if you have sensitive skin, ask a healthcare professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- For adults and children over 12 years, rub well on the affected area
- Repeat 3 to 4 times daily
- For children 12 years of age or younger, consult a doctor before use.
Other information
This product may cause allergic reaction in some individuals. Test on small area before use.
Inactive Ingredients
Dementholised Mint Oil, Diazolidinyl Urea, Eucalyptus Oil, FD&C Blue 1, Glycerin, Lavender Fragrance, Methyl Glucose Dioleate, Methylparaben, PVM/MA Decadiene Crosspolymer, Propylene Glycol, Propylparaben, Triethanolamine (TEA), Water.
Manufactured for Haw Par Healthcare Ltd., Singapore under licence from Haw Par Corporation Ltd.
Questions/Comments
(510) 887-1899
PRINCIPAL DISPLAY PANEL - 50g Tube Carton
NDC 66761-341-50
TIGER BALM®
TIGER BALM®
NECK &
SHOULDER
RUB
Pain Relieving
Cream
Non-Greasy
Soothing
Relief
VANISHING
SCENT
Soothing relief for stressed
and strained muscles
Net Wt. 1.76 oz (50g)
Haw Par Healthcare Ltd.