DESCRIPTION
Contains Lidocaine Hydrochloride. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure:
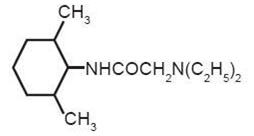
C14H22N2O
Each mL of Lido-K Lotion contains ACTIVE: Lidocaine HCl 30 mg in a lotion base of INACTIVES: Mineral Oil, Petrolatum, Cetyl Alcohol, Stearic Acid, Methylparaben, Propylparaben, Lexemul, Water, Sodium Hydroxide (50%), Aluminum Sulfate, and Calcium Acetate
CLINICAL PHARMACOLOGY
MECHANISM OF ACTION
Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action.
PHARMACOKINETICS
Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation in the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological / toxicological actions of these metabolites are similar to, but less potent than, those of Lidocaine. Approximately 90% of Lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6-dimethylaniline.
The plasma binding of Lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1-4 g of free base per mL, 60 to 80 percent of Lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein.
Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Studies of Lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which Lidocaine is metabolized, any condition that affects liver function may alter Lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect Lidocaine kinetics but may increase the accumulation of metabolites.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of Lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 g free base per mL. In the rhesus monkey, arterial blood levels of 18-21 g/ml have been shown to be threshold for convulsive activity.
CONTRAINDICATIONS
Traumatized mucosa, secondary bacterial infection of the area of proposed application and known hypersensitivity to any of the components. Lido-K is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to any other component of the product.
WARNINGS
For external use only. Not for ophthalmic use. Avoid contact with eyes, lips or mucous membranes. Do not use on areas of broken skin. If irritation or sensitivity occurs or infection appears, discontinue use. If swallowed, get medical help or contact a Poison Control Center right away.
Application of lidocaine to larger areas or for longer times than those recommended could result in sufficient absorption of lidocaine resulting in serious adverse effects.
Patients treated with class III anti-arrhythmic drugs (e.g., amiodarone, bretylium, sotalol, dofetilide) should be under close surveillance and ECG monitoring considered, because cardiac effects may be additive.
Lidocaine should be used with extreme caution in the presence of sepsis or severely traumatized mucosa in the area of application, since under such conditions there is the potential for rapid systemic absorption.
PRECAUTIONS
If irritation or sensitivity occurs or infection appears, discontinue treatment and institute appropriate therapy. Lido-K should be used with caution in ill, elderly, debilitated patients and children who may be more sensitive to the systemic effects of lidocaine.
CARCINOGENESIS, MUTAGENESIS AND IMPAIRMENT OF FERTILITY
Studies of Lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted.
USE IN PREGNANCY
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by Lidocaine. There are, however, no adequate and well controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering Lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
Lidocaine does pass very quickly through the placenta so doctors and experts suggest only using the anesthetic for established medical needs only as directed by your physician.
NURSING MOTHERS
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this drug is administered to a nursing mother. Your doctor and you will decide if the benefits outweigh the risk of using lidocaine.
PEDIATRIC USE
Dosage in pediatric patients would be reduced commensurate with age, body weight and physical condition.
If your child becomes very dizzy, excessively sleepy, or develops duskiness of the face or lips after applying lidocaine lotion, remove the lotion and contact your physician at once.
ADVERSE REACTIONS
During or immediately after treatment, the skin at the site of treatment may develop erythema or edema or maybe the locus of abnormal sensation.
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central nervous system
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest. Drowsiness following the administration of lidocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular system
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent or to other components in the formulation. Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
DOSAGE AND ADMINISTRATION
Apply a thin film to the affected area two or three times daily or as directed by a physician.
Caution should be exercised, particularly when employing large amounts, since the incidence of adverse effects is directly proportional to the total dose of local anesthetic agent administered.
