FEVERALL INFANTS- acetaminophen suppository
Actavis Pharma, Inc.
----------
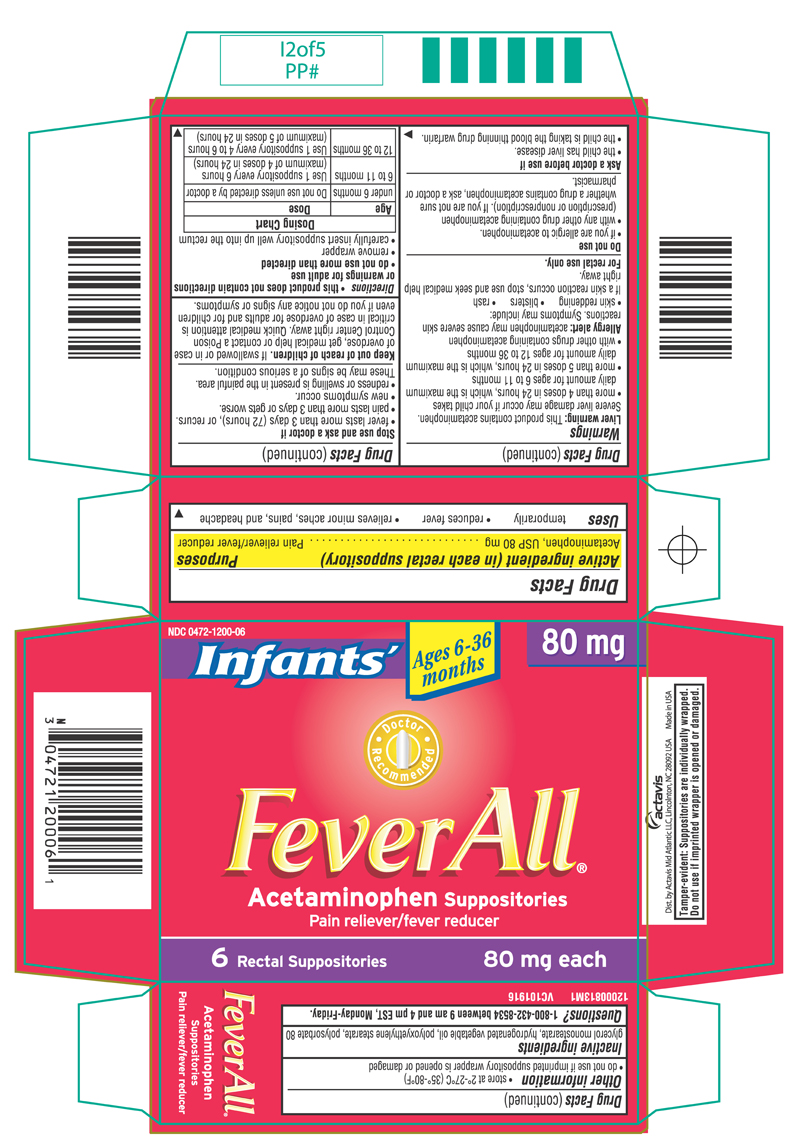
FeverAll ® (Acetaminophen Suppositories) INFANTS
WARNINGS
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 4 doses in 24 hours, which is the maximum daily amount for ages 6 to 11 months
- more than 5 doses in 24 hours, which is the maximum daily amount for ages 12 to 36 months
- with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
For rectal use only
DO NOT USE
- if you are allergic to acetaminophen.
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
ASK A DOCTOR BEFORE USE IF
- the child has liver disease.
- the child is taking the blood thinning drug warfarin.
STOP USE AND ASK A DOCTOR IF
- fever lasts more than 3 days (72 hours), or recurs.
- pain lasts more than 3 days or gets worse.
- new symptoms occur.
- redness or swelling is present in the painful area.
These may be signs of a serious condition.
KEEP OUT OF REACH OF CHILDREN
If swallowed or in case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical in case of overdose for adults and for children even if you do not notice any signs or symptoms.
DIRECTIONS
- this product does not contain directions or warnings for adult use
- do not use more than directed
- remove wrapper
- carefully insert suppository well up into the rectum
| Age | Dose |
| under 6 months | Do not use unless directed by a doctor |
| 6 to 11 months | Use 1 suppository every 6 hours (maximum of 4 doses in 24 hours) |
| 12 to 36 months | Use 1 suppository every 4 to 6 hours (maximum of 5 doses in 24 hours) |
OTHER INFORMATION
- store at 2º-27ºC (35º-80ºF)
- do not use if imprinted suppository wrapper is opened or damaged
| FEVERALL INFANTS
acetaminophen suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Actavis Pharma, Inc. (119723554) |
Revised: 3/2018
Document Id: 66c2a0a3-f43d-d46c-e053-2991aa0a7ef4
Set id: 2f1d555e-ad12-4b3c-9b2f-d824fed38400
Version: 9
Effective Time: 20180306
Actavis Pharma, Inc.