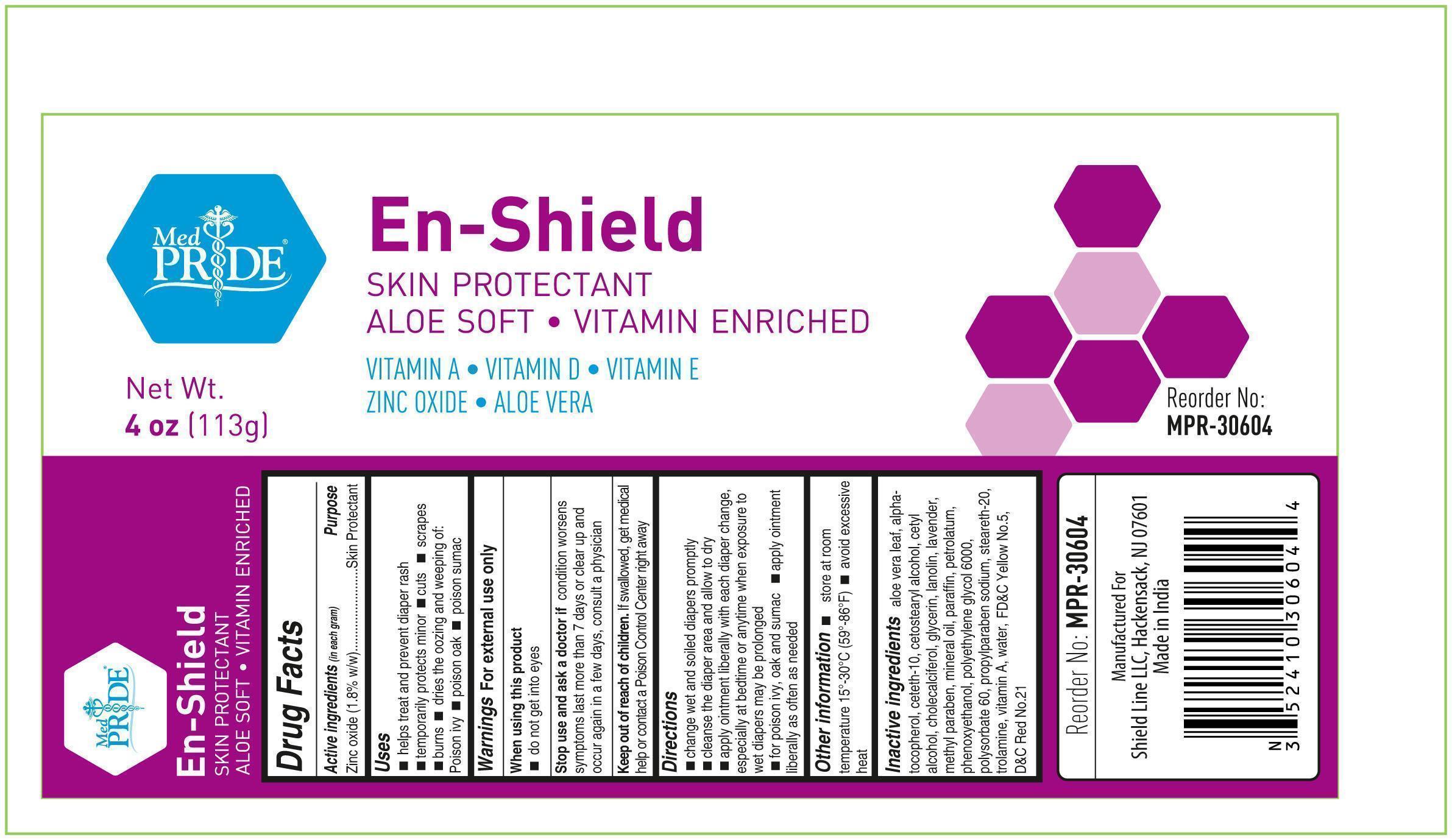
Uses
■ helps treat and prevent diaper rash ■ temporarily protects minor ■ cuts ■ scrapes ■ burns dries the oozing and weeping of: ■ Poison ivy ■ poison oak ■ poison sumac
Stop Use and ask a doctor if
if conditions worsen symptoms last more than 7 days or clear up and occur again in a few days, consult a physician
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
■ change wet and soiled diapers promptly ■ cleanse the diaper area and allow to dry ■ apply ointment liberally with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged ■ for poison ivy, oak and sumac ■ apply ointment liberally as needed
Inactive ingredients
aloe vera leaf, alpha-tocopherol, aeteth-10, cetostearyl alcohol, cetyl alcohol, cholecalciferol, glycerin, lanolin, lavender
methylparaben, mineral oil, paraffin, petrolatum, phenoxyethanol, polyethylene glycol 6000, polysorbate 60, propylparaben sodium
steareth-20, trolamine, vitamin A, water, FDC Yellow No.5, DC Red No.21