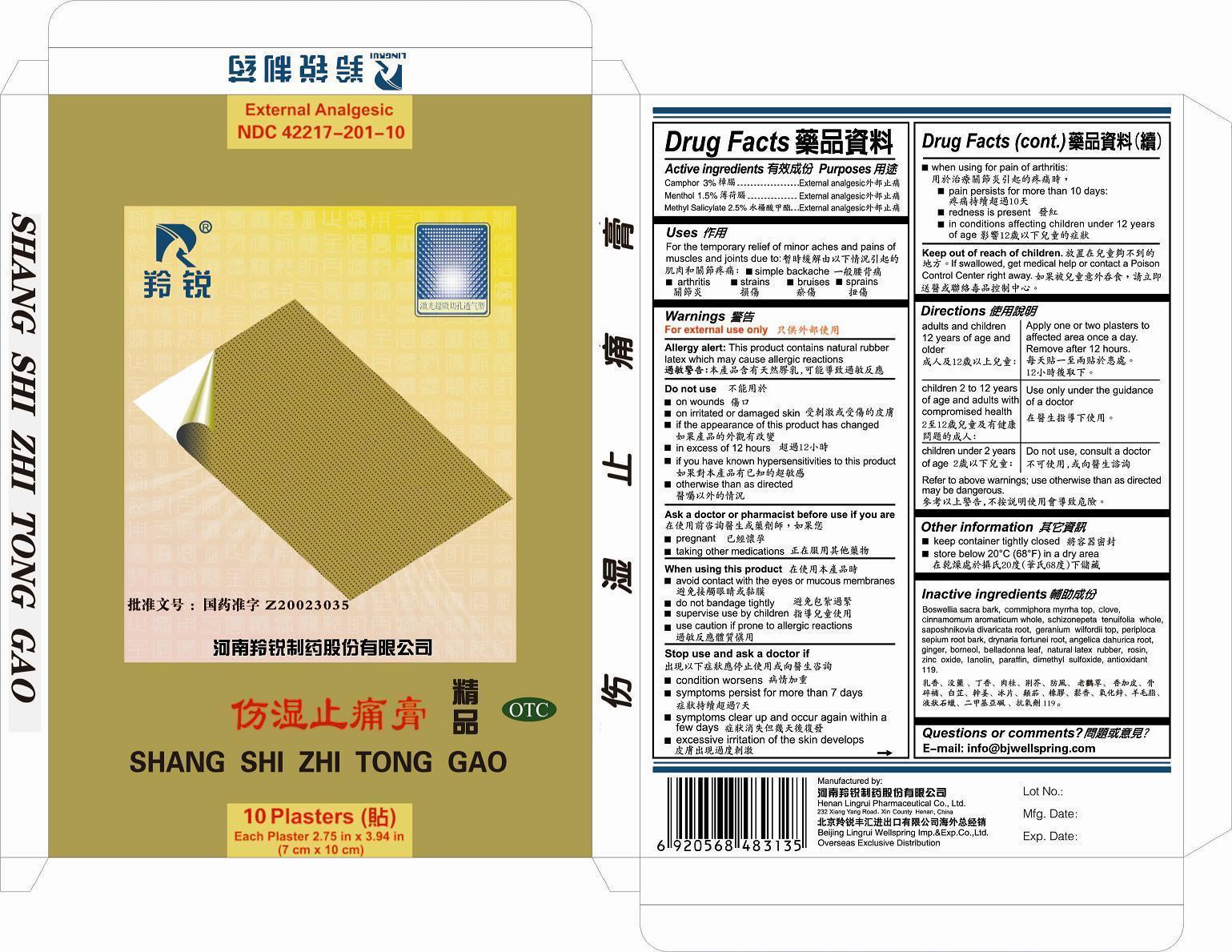
USES
To relieve the rheumatic pain and arthralgia by promoting blood circulation, used for rheumatoid arthristis muscular aches, sprain etc.
WARNINGS
For external use only.
Agergy Alert: This product contains natural rubber latex which may cause allergic reactions.
DO NOT USE
Do not use
on wounds.
on irritated or damaged skin
if the apperance of this product has changed
in excess of 12 hours
if you have know hypersensitivities to this product
otherwise than as directed
When using this product
avoid contact iwth the eyes or mucous membrances
do not bandage tightly
supervise use by children
use caution if prone to allergic reactions
Stop use and ask a doctor if
condition worsens
symptonms persist for more than 7 days
symptoms clear up and occur again within 1 few days
excessive irritation of the skin develops
when using for pain of arthritis
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years
Keep out of reach of children.
Keep out of reach of children. If swallowed, get medical help or Poison Control Center right away.
Adults and children 12 years of age and older: Apply one or two plasters to affected area once a day. Remove after 12 hours.
DIRECTIONB
Refer to the above warnings: use otherwise than as directed may be dangerous.
Adults and children 12 years of age and older: Apply one or two plasters to affected area once a day. Remove after 12 hours.
Children 2 to 12 years of age and adults with compromised health: Use only under the guidance of a doctor.
Children under 2 years of age: Do not use, consult a doctor.
Refer to the above warnings: use otherwise than as directed may be dangerous.
OTHER INFORMATION
Keep container tightly closed.
Store below 20 degree centigrade (68 E F) in a dry area.
INACTIVE INGREDIENTS
BOSWELLIA SACRA BARK, COMMIPHORA MYRRHA TOP, CLOVE, CINNAMOMUM AROMATICUM WHOLE, SCHIZONEPETA TENUIFOLIA WHOLE, SAPOSHNIKOVIA DIVARICATA ROOT, GERANIUM WILFORDII TOP, PERIPLOCA SEPIUM ROOT BARK, DRYNARIA FORTUNEI ROOT, ANGELICA DAHURICA ROOT, GINGER, BORNEOL, BELLADONNA LEAF, NATURAL LATEX RUBBER, ROSIN, ZINC OXIDE, LANOLIN, PARAFFIN, DIMETHYL SULFOXIDE, ANTIOXIDANT 119