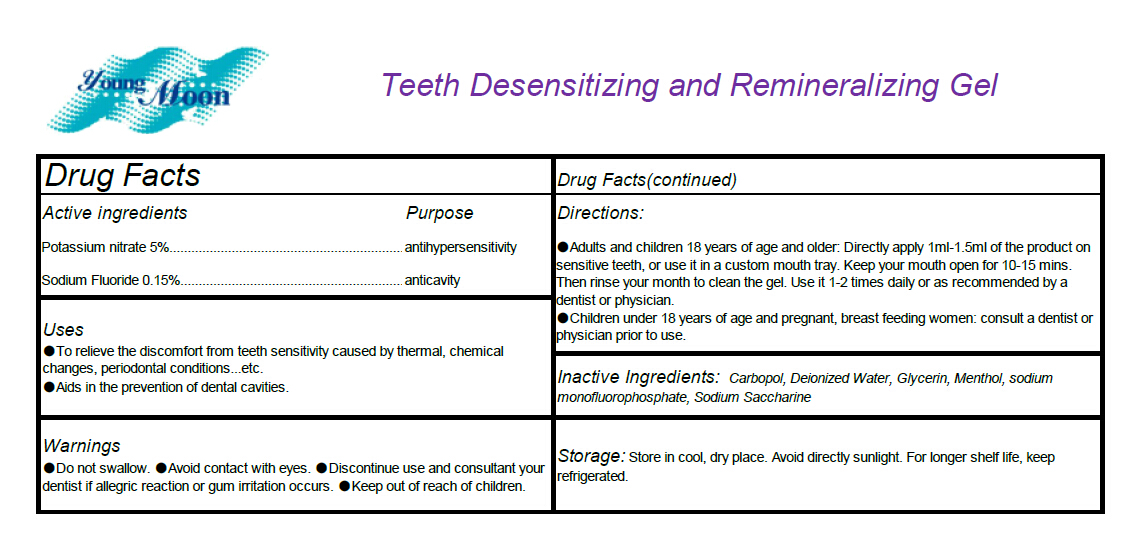
Uses
To relieve the discomfort from teeth sensitivity caused by thermal, chemical changes, periodontal
conditions...etc. Aids in the prevention of dental cavities.
Warnings
Do not swallow. Avoid contact with eyes. Discontinue use and consultant your dentist if allegric
reaction or gum irritation occurs.
Directions
Adults and children 18 years of age and older: Directly apply 1ml-1.5ml of the product on
sensitive teeth, or use it in a custom mouth tray. Keep your mouth open for 10-15 mins. Then rinse
your month to clean the gel. Use it 1-2 times daily or as recommended by a dentist or physician.
Children under 18 years of age and pregnant, breast feeding women: consult a dentist or
physician prior to use.