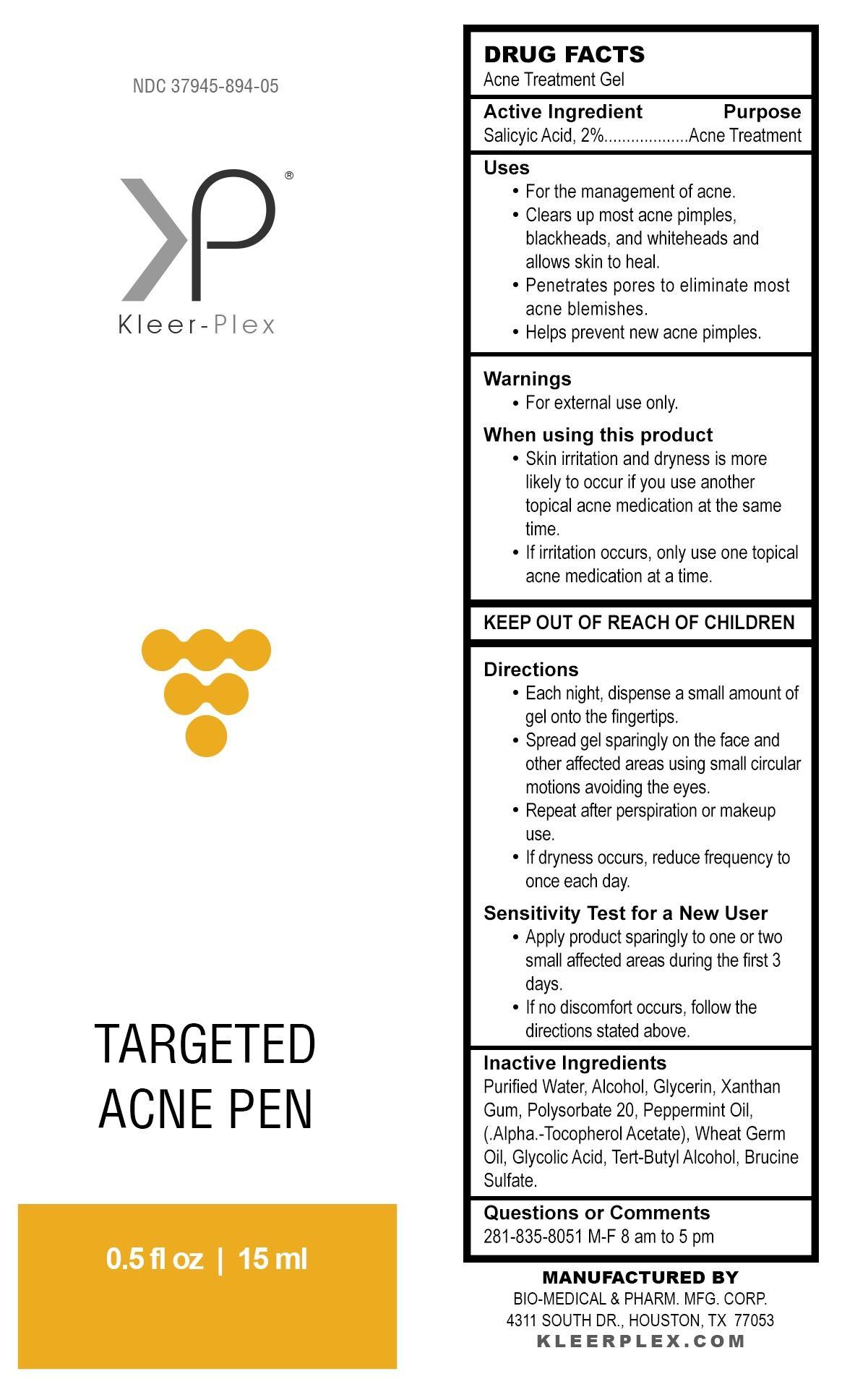
Use
For the management of acne.
Clears up most acne pimples, blackheads, and whiteheads and allows skin to heal.
Penetrates pores to eliminate most acne blemishes.
Helps prevent new acne pimples.
When using this product
Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time.
If irritation occurs, only use one topical acne medication at a time.
Directions
Each night, dispense a small amount of gel onto the fingertips.
Spread gel sparingly on the face and other affected areas using small circular motions avoiding the eyes.
Repeat after perspiration or makeup use.
If dryness occurs, reduce frequency to once each day.
Sensitivity test for a new user
Apply product sparingly to one or two small affected areas during the first 3 days.
If no discomfort occurs, follow the directions stated above.