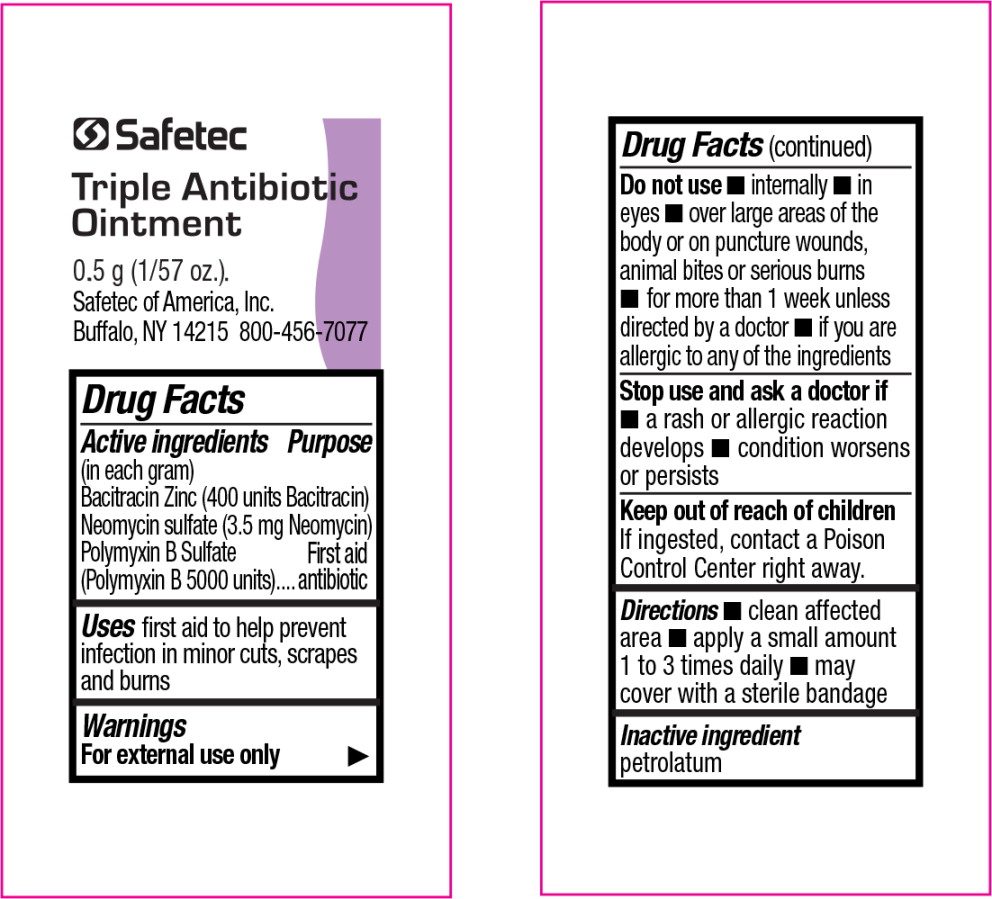
Active Ingredient (in each gram)
Bacitracin Zinc (400 units Bacitracin)
Neomycin Sulfate 5 mg (equivalent to 3.5 mg of Neomycin base)
Polymyxin-B Sulfate 5000 units
Do not use:
- in the eyes
- internally
- over large areas of the body
- if you are allergic to any of the ingredients
- longer than 1 week unless directed by a doctor
Stop use and ask a doctor if:
- the condition persists or gets worse
- a rash or other allergic reaction develops
Keep this and all drugs out of the reach of children.
In case of accidental ingestion, seek professional assistance or contact a poison control center immediately.
Directions
- Clean the affected area
- Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- May be covered with a sterile bandage
Questions or Comments? 1-800-456-7077
Manufactured by
SAFETEC OF AMERICA, Inc.
Buffalo, NY 14215 • 800-456-7077
www.safetec.com