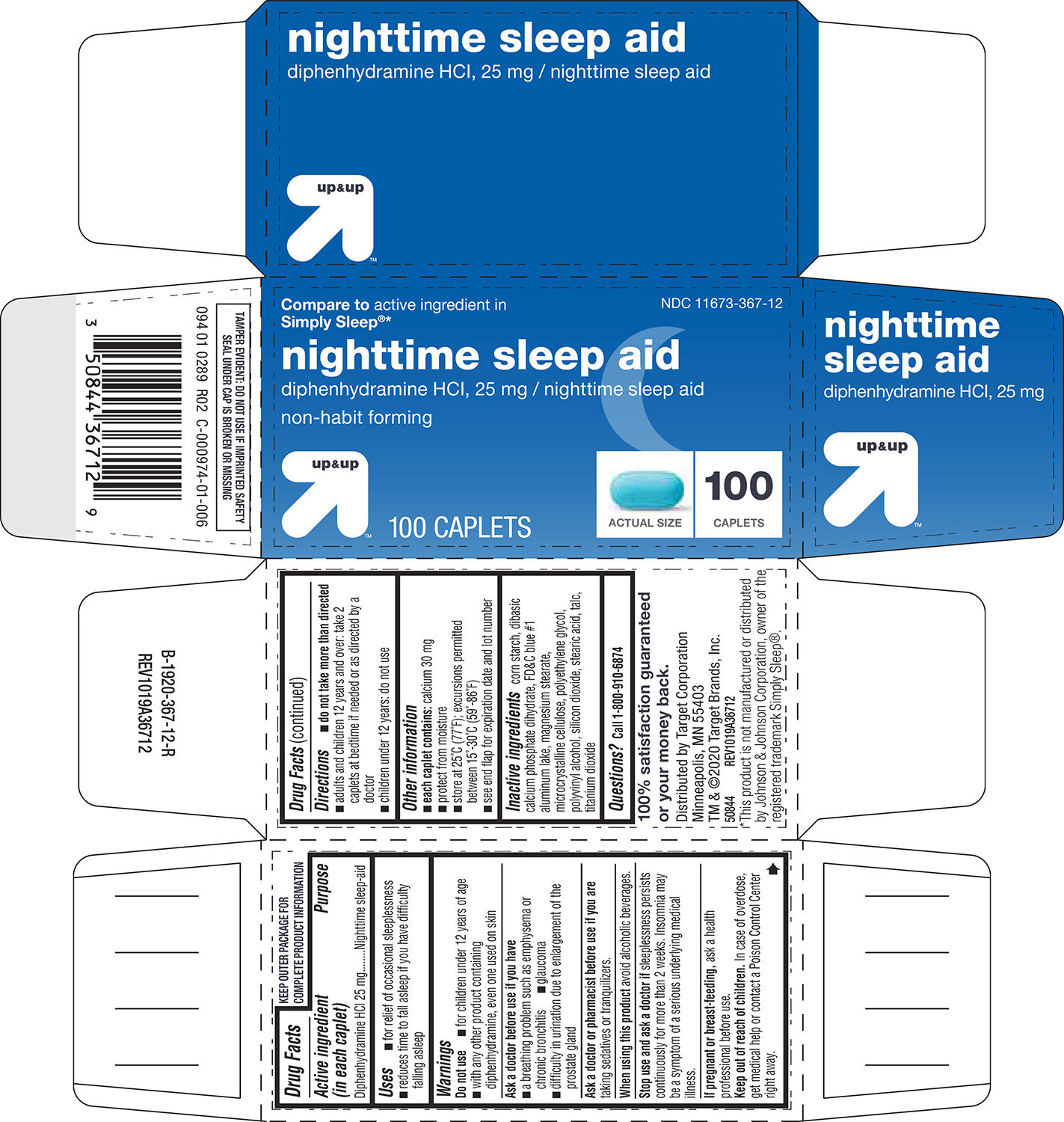
Uses
- for relief of occasional sleeplessness
- reduces time to fall asleep if you have difficulty falling asleep
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- do not take more than directed
- adults and children 12 years and over: take 2 caplets at bedtime if needed or as directed by a doctor
- children under 12 years: do not use
Other information
- each caplet contains: calcium 30 mg
- protect from moisture
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, stearic acid, talc, titanium dioxide
Principal Display Panel
Compare to active ingredient in
Simply Sleep®*
NDC 11673-367-12
nighttime sleep aid
diphenhydramine HCI, 25 mg / nighttime sleep aid
non-habit forming
up&up™
100 CAPLETS
ACTUAL SIZE
100
CAPLETS
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING
100% satisfaction guaranteed
or your money back.
Distributed by Target Corporation
Minneapolis, MN 55403
TM & ©2020 Target Brands, Inc.
50844 REV1019A36712
*This product is not manufactured or distributed
by Johnson & Johnson Corporation, owner of the
registered trademark Simply Sleep®.
Target 44-367