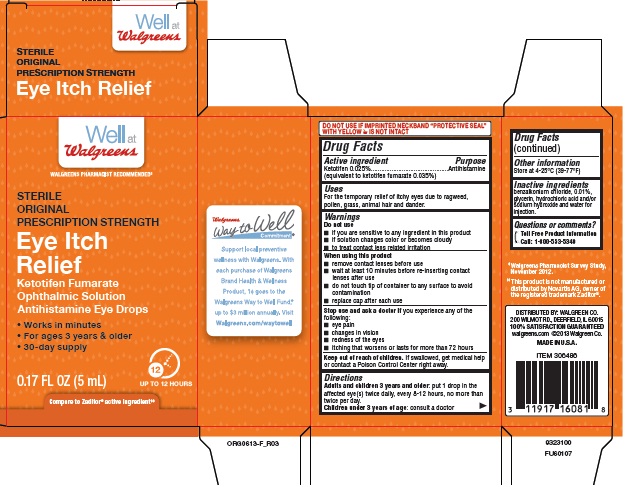
Warnings
Do not use
- •
- if you are sensitive to any ingredient in this product
- •
- if solution changes color or becomes cloudy
- •
- to treat contact lens related irritation
When using this product
- •
- remove contact lenses before use
- •
- wait at least 10 minutes before re-inserting contact lenses after use
- •
- do not touch tip of container to any surface to avoid contamination
- •
- replace cap after each use
Directions
- •
- Adults and children 3 years and older: put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day.
- •
- Children under 3 years of age: consult a doctor
Inactive ingredients
benzalkonium chloride 0.01%, glycerin, hydrochloric acid and/or sodium hydroxide and water for injection.
Questions or comments?
Toll Free Product Information
Call: 1-800-553-5340
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
walgreens.com ©2013 Walgreen Co.
MADE IN U.S.A.
ITEM 306486
9323100
FU60107
Principal Display Panel
Well at
Walgreens
WALGREENS PHARMACIST RECOMMENDED‡
STERILE
ORIGINAL
PRESCRIPTION STRENGTH
Eye Itch
Relief
Ketotifen Fumarate
Ophthalmic Solution
Antihistamine Eye Drops
- •
- Works in minutes
- •
- For ages 3 years & older
- •
- 30-day supply
0.17 FL OZ (5 mL)
12 UP TO 12 HOURS
Compare to Zaditor® active ingredient‡‡